Patient trajectories after diagnosis of diffuse large B-cell lymphoma-a multistate modelling approach to estimate the chance of lasting remission
- PMID: 35999271
- PMCID: PMC9596493
- DOI: 10.1038/s41416-022-01931-2
Patient trajectories after diagnosis of diffuse large B-cell lymphoma-a multistate modelling approach to estimate the chance of lasting remission
Abstract
Background: Achieving lasting remission for at least 2 years is a good indicator for favourable prognosis long term after Diffuse large B-cell lymphoma (DLBCL). The aim of this study was to provide real-world probabilities, useful in risk communication and clinical decision-making, of the chance for lasting remissions by clinical characteristics.
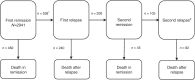
Methods: DLBCL patients in remission after primary treatment recorded in the Swedish Lymphoma register 2007-2014 (n = 2941) were followed for relapse and death using multistate models to study patient trajectories. Flexible parametric models were used to estimate transition rates.
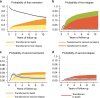
Results: At 2 years, 80.7% (95% CI: 79.0-82.2) of the patients were predicted to remain in remission and 13.2% (95% CI: 11.9-14.6) to have relapsed. The relapse risk peaked at 7 months, and the annual decline of patients in remission stabilised after 2 years. The majority of patients in the second remission transitioned into a new relapse. The probability of a lasting remission was reduced by 20.4% units for patients with IPI 4-5 compared to patients with IPI 0-1, and time in remission was shortened by 3.5 months.
Conclusion: The long-term prognosis was overall favourable with 80% achieving durable first remissions. However, prognosis varied by clinical subgroups and relapsing patients seldom achieved durable second remissions.
© 2022. The Author(s).
Conflict of interest statement
Sara Ekberg, SH, Sandra Eloranta and KES are part of a research collaboration between Karolinska Institutet and Janssen Pharmaceutica NV for which Karolinska Institutet has received grant support. KES has received Grant support from Janssen Pharmaceuticals and Takeda. MJ has received research support from Janssen Pharmaceuticals, Roche, Gilead, AstraZeneca and Abbvie and honoraria from Janssen Pharmaceuticals, Incyte, Gilead, AstraZeneca, Abbvie and Orion. MC has received consultancy fees from StataCorp LLC.
Figures


References
-
- Coiffier B, Thieblemont C, Van Den Neste E, Lepeu G, Plantier I, Castaigne S, et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d’Etudes des Lymphomes de l’Adulte. Blood. 2010;116:2040–5. doi: 10.1182/blood-2010-03-276246. - DOI - PMC - PubMed
-
- Pfreundschuh M, Kuhnt E, Trümper L, Osterborg A, Trneny M, Shepherd L, et al. CHOP-like chemotherapy with or without rituximab in young patients with good-prognosis diffuse large-B-cell lymphoma: 6-year results of an open-label randomised study of the MabThera International Trial (MInT) Group. Lancet Oncol. 2011;12:1013–22. doi: 10.1016/S1470-2045(11)70235-2. - DOI - PubMed
-
- Pfreundschuh M, Schubert J, Ziepert M, Schmits R, Mohren M, Lengfelder E, et al. Six versus eight cycles of bi-weekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20+ B-cell lymphomas: a randomised controlled trial (RICOVER-60) Lancet Oncol. 2008;9:105–16. doi: 10.1016/S1470-2045(08)70002-0. - DOI - PubMed
-
- Pfreundschuh M, Trumper L, Osterborg A, Pettengell R, Trneny M, Imrie K, et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 2006;7:379–91. doi: 10.1016/S1470-2045(06)70664-7. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

