Cytoskeletal assembly in axonal outgrowth and regeneration analyzed on the nanoscale
- PMID: 35999340
- PMCID: PMC9399097
- DOI: 10.1038/s41598-022-18562-5
Cytoskeletal assembly in axonal outgrowth and regeneration analyzed on the nanoscale
Abstract
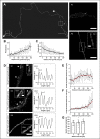
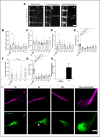
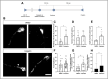
The axonal cytoskeleton is organized in a highly periodic structure, the membrane-associated periodic skeleton (MPS), which is essential to maintain the structure and function of the axon. Here, we use stimulated emission depletion microscopy of primary rat cortical neurons in microfluidic chambers to analyze the temporal and spatial sequence of MPS formation at the distal end of growing axons and during regeneration after axotomy. We demonstrate that the MPS does not extend continuously into the growing axon but develops from patches of periodic βII-spectrin arrangements that grow and coalesce into a continuous scaffold. We estimate that the underlying sequence of assembly, elongation, and subsequent coalescence of periodic βII-spectrin patches takes around 15 h. Strikingly, we find that development of the MPS occurs faster in regenerating axons after axotomy and note marked differences in the morphology of the growth cone and adjacent axonal regions between regenerating and unlesioned axons. Moreover, we find that inhibition of the spectrin-cleaving enzyme calpain accelerates MPS formation in regenerating axons and increases the number of regenerating axons after axotomy. Taken together, we provide here a detailed nanoscale analysis of MPS development in growing axons.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Lukinavičius G, et al. Fluorogenic probes for live-cell imaging of the cytoskeleton. Nat. Methods. 2014;11:731–733. - PubMed
-
- D’Este E, Kamin D, Göttfert F, El-Hady A, Hell SW. STED Nanoscopy reveals the ubiquity of subcortical cytoskeleton periodicity in living neurons. Cell Rep. 2015;10:1246–1251. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

