Efficacy of SCF drug conjugate targeting c-KIT in gastrointestinal stromal tumor
- PMID: 35999600
- PMCID: PMC9400206
- DOI: 10.1186/s12916-022-02465-3
Efficacy of SCF drug conjugate targeting c-KIT in gastrointestinal stromal tumor
Abstract
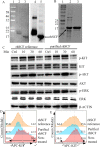
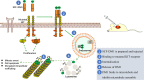
Background: Gastrointestinal stromal tumor (GIST) is a rare type of cancer that occurs in the gastrointestinal tract. The majority of GIST cases carry oncogenic forms of KIT, the receptor for stem cell factor (SCF). Small molecule kinase inhibitor imatinib is effective in prolonging the survival of GIST patients by targeting KIT. However, drug resistance often develops during the therapeutic treatment. Here, we produced a SCF-emtansine drug conjugate (SCF-DM1) with favorable drug efficacy towards GIST cells.
Methods: Recombinant human SCF (rhSCF) was expressed in E. coli cells and further purified with Ni-NTA Sepharose and Phenyl Sepharose. It was then conjugated with DM1, and the conjugated product SCF-DM1 was evaluated using in vitro cell-based assays and in vivo xenograft mouse model.
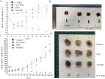
Results: SCF-DM1 was effective in inhibiting imatinib-sensitive and -resistant GIST cell lines and primary tumor cells, with IC50 values of < 30 nM. It induced apoptosis and cell cycle arrest in GIST cells. In xenograft mouse model, SCF-DM1 showed favorable efficacy and safety profiles.
Conclusions: rhSCF is a convenient and effective vector for drug delivery to KIT positive GIST cells. SCF-DM1 is an effective drug candidate to treat imatinib-sensitive and -resistant GIST.
Keywords: DM1; GIST; SCF; Targeted therapy.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





Similar articles
-
A novel anti-c-Kit antibody-drug conjugate to treat wild-type and activating-mutant c-Kit-positive tumors.Mol Oncol. 2022 Mar;16(6):1290-1308. doi: 10.1002/1878-0261.13084. Epub 2021 Aug 29. Mol Oncol. 2022. PMID: 34407310 Free PMC article.
-
Combination of Imatinib Mesylate and AKT Inhibitor Provides Synergistic Effects in Preclinical Study of Gastrointestinal Stromal Tumor.Clin Cancer Res. 2017 Jan 1;23(1):171-180. doi: 10.1158/1078-0432.CCR-16-0529. Epub 2016 Jul 1. Clin Cancer Res. 2017. PMID: 27370604 Free PMC article.
-
Dual Targeting of Insulin Receptor and KIT in Imatinib-Resistant Gastrointestinal Stromal Tumors.Cancer Res. 2017 Sep 15;77(18):5107-5117. doi: 10.1158/0008-5472.CAN-17-0917. Epub 2017 Jul 31. Cancer Res. 2017. PMID: 28760855
-
Imatinib mesylate: in the treatment of gastrointestinal stromal tumours.Drugs. 2003;63(5):513-22; discussion 523-4. doi: 10.2165/00003495-200363050-00005. Drugs. 2003. PMID: 12600228 Review.
-
Novel Insights into the Treatment of Imatinib-Resistant Gastrointestinal Stromal Tumors.Target Oncol. 2017 Jun;12(3):277-288. doi: 10.1007/s11523-017-0490-9. Target Oncol. 2017. PMID: 28478525 Review.
Cited by
-
Characterization of a Human Gastrointestinal Stromal Tumor Cell Line Established by SV40LT-Mediated Immortalization.Int J Mol Sci. 2023 Sep 4;24(17):13640. doi: 10.3390/ijms241713640. Int J Mol Sci. 2023. PMID: 37686448 Free PMC article.
-
Leukemogenic SHP2 mutations lead to erythropoietin independency of HCD-57 cells: a novel model for preclinical research of SHP2-mutant JMML.Exp Hematol Oncol. 2023 Feb 20;12(1):20. doi: 10.1186/s40164-023-00379-1. Exp Hematol Oncol. 2023. PMID: 36805832 Free PMC article.
-
The cellular triumvirate: fibroblasts entangled in the crosstalk between cancer cells and immune cells.Front Immunol. 2024 Jan 18;14:1337333. doi: 10.3389/fimmu.2023.1337333. eCollection 2023. Front Immunol. 2024. PMID: 38313431 Free PMC article. Review.
-
Advances in immunology and immunotherapy for mesenchymal gastrointestinal cancers.Mol Cancer. 2023 Apr 18;22(1):71. doi: 10.1186/s12943-023-01770-6. Mol Cancer. 2023. PMID: 37072770 Free PMC article. Review.
-
TATA-box-binding protein-associated factor 15 is a novel biomarker that promotes cell proliferation and migration in gastrointestinal stromal tumor.World J Gastroenterol. 2023 May 21;29(19):2932-2949. doi: 10.3748/wjg.v29.i19.2932. World J Gastroenterol. 2023. PMID: 37274797 Free PMC article.
References
-
- Rubin BP, Blanke Cd Fau - Demetri GD, Demetri Gd Fau - Dematteo RP, Dematteo Rp Fau - Fletcher CDM, Fletcher Cd Fau - Goldblum JR, Goldblum Jr Fau - Lasota J, Lasota J Fau - Lazar A, Lazar A Fau - Maki RG, Maki Rg Fau - Miettinen M, Miettinen M Fau - Noffsinger A et al: Protocol for the examination of specimens from patients with gastrointestinal stromal tumor. (1543–2165 (Electronic)). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

