Head and Neck Wound Reconstruction Using Biodegradable Temporizing Matrix Versus Collagen-Chondroitin Silicone Bilayer
- PMID: 36000010
- PMCID: PMC9361342
Head and Neck Wound Reconstruction Using Biodegradable Temporizing Matrix Versus Collagen-Chondroitin Silicone Bilayer
Abstract
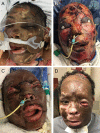
Background: Head and neck reconstruction is challenging because of the functional requirements of movement, sensation, and cosmesis of this highly visible region. This study is the first to compare Novosorb biodegradable temporizing matrix (BTM) and Integra collagen-chondroitin silicone (CCS) skin substitutes for reconstruction of soft tissue head and neck wounds.
Methods: This retrospective review included adults who underwent wound reconstruction of the head/neck with either BTM or CCS between 2015 and 2020. Patient-level data, complications, and closure rates were compared.
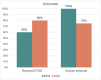
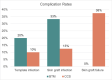
Results: The review identified 15 patients: 5 who received BTM and 10 who received CCS. Mean age at dermal template placement was 55 (range, 28-79) years. Race, sex, smoking status, medical comorbidities, defect size, radiation history, prior surgeries, and follow-up time were not significantly different between groups. Wound etiologies for BTM and CCS included burn (40% vs 60%), trauma (20% vs 20%), surgical wounds (20% vs 20%), and skin cancer (20% vs 0%), respectively (P = .026). Skin grafts were placed in 8 (80%) wounds after CCS placement, compared with 3 (60%) after BTM (P = .670). Template reapplication was required in 2 (40%) BTM wounds and 3 (30%) CCS wounds (P = 1.0). Infection, hematoma, and seroma were comparable between groups, although skin graft failure was higher in the CCS group at 3 (37.5%) compared with 0 for BTM (P = .506). More secondary procedures were required after CCS placement (CCS, 1.9 ± 2.2; BTM, 0.9 ± 0.8; P = .090). Definitive closure in patients not lost to follow-up occurred in 4 (100%) BTM and 6 (75%) CCS cases (P = 1.0).
Conclusions: Head and neck wounds treated with BTM had comparable closure and complication rates as CCS bilayer and required fewer secondary procedures and skin grafts. These findings suggest that BTM is safe and efficacious for application in head and neck wounds and may be considered as an economical alternative.
Keywords: Integra; Novosorb; biodegradable temporizing matrix; dermal substitute; head and neck; wound healing.
© 2022, HMP Global. All rights reserved. Reproduction in whole or in part prohibited. Content may not be reproduced in any form without written permission. Rights, Permission, Reprint, and Translation information is available at www.hmpglobal.com.
Conflict of interest statement
Disclosures: KC and JC are paid consultants for Polynovo Biomaterials but were not involved in data collection or analysis. None of the other authors have significant conflicts of interest with any companies or organizations whose products or services may be discussed in this article.
Figures


Similar articles
-
Performance of biodegradable temporizing matrix vs collagen-chondroitin silicone bilayer dermal regeneration substitutes in soft tissue wound healing: a retrospective analysis.Wounds. 2022 Apr;34(4):106-115. doi: 10.25270/wnds/2022.106115. Wounds. 2022. PMID: 35452408
-
Upper Extremity Wounds Treated with Biodegradable Temporizing Matrix versus Collagen-Chondroitin Silicone Bilayer.J Hand Microsurg. 2022 Jun 1;15(5):340-350. doi: 10.1055/s-0042-1749077. eCollection 2023 Dec. J Hand Microsurg. 2022. PMID: 38152680 Free PMC article.
-
Reconstruction of Complex Upper Extremity Wounds With Novosorb Biodegradable Temporizing Matrix Versus Integra Collagen-Chondroitin Silicone: A Cost Analysis.Eplasty. 2024 Jun 18;24:e38. eCollection 2024. Eplasty. 2024. PMID: 39224413 Free PMC article.
-
The use of NovoSorb biodegradable temporising matrix in wound management: a literature review and case series.J Wound Care. 2023 Aug 2;32(8):470-478. doi: 10.12968/jowc.2023.32.8.470. J Wound Care. 2023. PMID: 37572341 Review.
-
The Efficacy of Biodegradable Temporising Matrix for Upper Limb Reconstruction: A Systematic Review and Meta-Analysis.Cureus. 2024 Dec 19;16(12):e75994. doi: 10.7759/cureus.75994. eCollection 2024 Dec. Cureus. 2024. PMID: 39711936 Free PMC article. Review.
Cited by
-
Approaches to Cheek Reconstruction following Mohs Surgery.Semin Plast Surg. 2024 Nov 4;38(4):321-325. doi: 10.1055/s-0044-1791223. eCollection 2024 Nov. Semin Plast Surg. 2024. PMID: 39697409 Review.
-
Novosorb® BTM- history, production and application in challenging wounds.Front Bioeng Biotechnol. 2024 Nov 13;12:1450973. doi: 10.3389/fbioe.2024.1450973. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 39634098 Free PMC article. Review.
-
Biodegradable Temporising matrix in the reconstruction of complex wounds: A systematic review and meta-analysis.Int Wound J. 2024 Oct;21(10):e70025. doi: 10.1111/iwj.70025. Int Wound J. 2024. PMID: 39401977 Free PMC article.
-
Treatment of Complex Wounds with NovoSorb® Biodegradable Temporising Matrix (BTM)-A Retrospective Analysis of Clinical Outcomes.J Pers Med. 2022 Dec 3;12(12):2002. doi: 10.3390/jpm12122002. J Pers Med. 2022. PMID: 36556223 Free PMC article.
-
Matrix for Mucosal Regeneration in Transoral Glossectomy for Squamous Cell Carcinoma: Objective and Subjective Functional Evaluation.Curr Oncol. 2023 Jan 17;30(2):1354-1362. doi: 10.3390/curroncol30020104. Curr Oncol. 2023. PMID: 36826065 Free PMC article.
References
-
- Brusselaers N, Pirayesh A, Hoeksema H, et al. . Skin replacement in burn wounds. J Trauma. 2010;68(2):490-501. doi:10.1097/TA.0b013e3181c9c074 - DOI - PubMed
-
- Chou TD, Chen SL, Lee TW, et al. . Reconstruction of burn scar of the upper extremities with artificial skin. Plast Reconstr Surg. 2001;108(2). doi:10.1097/00006534-200108000-0001510.1097/00006534-200108000-00015 - DOI - DOI - PubMed
-
- Ryan CM, Schoenfeld DA, Malloy M, Schulz JT 3rd, Sheridan RL, Tompkins RG. Use of Integra artificial skin is associated with decreased length of stay for severely injured adult burn survivors. J Burn Care Rehabil. 2002;23(5):311-317. doi:10.1097/00004630-200209000-0000210.1097/00004630-200209000-00002 - DOI - DOI - PubMed
-
- Heimbach DM, Warden GD, Luterman A, et al. . Multicenter postapproval clinical trial of Integra dermal regeneration template for burn treatment. J Burn Care Rehabil. 2003;24(1):42-48. doi:10.1097/00004630-200301000-0000910.1097/00004630-200301000-00009 - DOI - DOI - PubMed
-
- Schiavon M, Francescon M, Drigo D, et al. . The use of integra dermal regeneration template versus flaps for reconstruction of full-thickness scalp defects involving the calvaria: a cost-benefit analysis. Aesthetic Plast Surg. 2016;40(6):901-907. doi:10.1007/s00266-016-0703-010.1007/s00266-016-0703-0 - DOI - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
