Luteal phase support for women trying to conceive by intrauterine insemination or sexual intercourse
- PMID: 36000704
- PMCID: PMC9400390
- DOI: 10.1002/14651858.CD012396.pub2
Luteal phase support for women trying to conceive by intrauterine insemination or sexual intercourse
Abstract
Background: Ovulation induction may impact endometrial receptivity due to insufficient progesterone secretion. Low progesterone is associated with poor pregnancy outcomes.
Objectives: To assess the effectiveness and safety of luteal phase support (LPS) in infertile women trying to conceive by intrauterine insemination or by sexual intercourse.
Search methods: We searched the Cochrane Gynaecology and Fertility Group Specialised Register, CENTRAL, MEDLINE, Embase, PsycINFO, LILACS, trial registries for ongoing trials, and reference lists of articles (from inception to 25 August 2021).
Selection criteria: Randomised controlled trials (RCTs) of LPS using progestogen, human chorionic gonadotropin (hCG), or gonadotropin-releasing hormone (GnRH) agonist supplementation in IUI or natural cycle.
Data collection and analysis: We used standard methodological procedures expected by Cochrane. Our primary outcomes were live birth rate/ongoing pregnancy rate (LBR/OPR) and miscarriage. MAIN RESULTS: We included 25 RCTs (5111 participants). Most studies were at unclear or high risk of bias. We graded the certainty of evidence as very low to low. The main limitations of the evidence were poor reporting and imprecision. 1. Progesterone supplement versus placebo or no treatment We are uncertain if vaginal progesterone increases LBR/OPR (risk ratio (RR) 1.10, 95% confidence interval (CI) 0.81 to 1.48; 7 RCTs; 1792 participants; low-certainty evidence) or decreases miscarriage per pregnancy compared to placebo or no treatment (RR 0.70, 95% CI 0.40 to 1.25; 5 RCTs; 261 participants). There were no data on LBR or miscarriage with oral stimulation. We are uncertain if progesterone increases LBR/OPR in women with gonadotropin stimulation (RR 1.24, 95% CI 0.80 to 1.92; 4 RCTs; 1054 participants; low-certainty evidence) and oral stimulation (clomiphene citrate or letrozole) (RR 0.97, 95% CI 0.58 to 1.64; 2 RCTs; 485 participants; low-certainty evidence). One study reported on OPR in women with gonadotropin plus oral stimulation; the evidence from this study was uncertain (RR 0.73, 95% CI 0.37 to 1.42; 1 RCT; 253 participants; low-certainty evidence). Given the low certainty of the evidence, it is unclear if progesterone reduces miscarriage per clinical pregnancy in any stimulation protocol (RR 0.68, 95% CI 0.24 to 1.91; 2 RCTs; 102 participants, with gonadotropin; RR 0.67, 95% CI 0.30 to 1.50; 2 RCTs; 123 participants, with gonadotropin plus oral stimulation; and RR 0.53, 95% CI 0.25 to 1.14; 2 RCTs; 119 participants, with oral stimulation). Low-certainty evidence suggests that progesterone in all types of ovarian stimulation may increase clinical pregnancy compared to placebo (RR 1.38, 95% CI 1.10 to 1.74; 7 RCTs; 1437 participants, with gonadotropin; RR 1.40, 95% CI 1.03 to 1.90; 4 RCTs; 733 participants, with gonadotropin plus oral stimulation (clomiphene citrate or letrozole); and RR 1.44, 95% CI 1.04 to 1.98; 6 RCTs; 1073 participants, with oral stimulation). 2. Progesterone supplementation regimen We are uncertain if there is any difference between 300 mg and 600 mg of vaginal progesterone for OPR and multiple pregnancy (RR 1.58, 95% CI 0.81 to 3.09; 1 RCT; 200 participants; very low-certainty evidence; and RR 0.50, 95% CI 0.05 to 5.43; 1 RCT; 200 participants, very low-certainty evidence, respectively). No other outcomes were reported for this comparison. There were three different comparisons between progesterone regimens. For OPR, the evidence is very uncertain for intramuscular (IM) versus vaginal progesterone (RR 0.59, 95% CI 0.34 to 1.02; 1 RCT; 225 participants; very low-certainty evidence); we are uncertain if there is any difference between oral and vaginal progesterone (RR 1.25, 95% CI 0.70 to 2.22; 1 RCT; 150 participants; very low-certainty evidence) or between subcutaneous and vaginal progesterone (RR 1.05, 95% CI 0.54 to 2.05; 1 RCT; 246 participants; very low-certainty evidence). We are uncertain if IM or oral progesterone reduces miscarriage per clinical pregnancy compared to vaginal progesterone (RR 0.75, 95% CI 0.43 to 1.32; 1 RCT; 81 participants and RR 0.58, 95% CI 0.11 to 3.09; 1 RCT; 41 participants, respectively). Clinical pregnancy and multiple pregnancy were reported for all comparisons; the evidence for these outcomes was very uncertain. Only one RCT reported adverse effects. We are uncertain if IM route increases the risk of adverse effects when compared with the vaginal route (RR 9.25, 95% CI 2.21 to 38.78; 1 RCT; 225 participants; very low-certainty evidence). 3. GnRH agonist versus placebo or no treatment No trials reported live birth. The evidence is very uncertain about the effect of GnRH agonist in ongoing pregnancy (RR 1.10, 95% CI 0.70 to 1.74; 1 RCT; 291 participants, very low-certainty evidence), miscarriage per clinical pregnancy (RR 0.73, 95% CI 0.26 to 2.10; 2 RCTs; 79 participants, very low-certainty evidence) and clinical pregnancy (RR 1.00, 95% CI 0.68 to 1.47; 2 RCTs; 340 participants; very low-certainty evidence), and multiple pregnancy (RR 0.28, 95% CI 0.11 to 0.70; 2 RCTs; 126 participants). 4. GnRH agonist versus vaginal progesterone The evidence for the effect of GnRH agonist injection on clinical pregnancy is very uncertain (RR 1.00, 95% CI 0.51 to 1.95; 1 RCT; 242 participants). 5. HCG injection versus no treatment The evidence for the effect of hCG injection on clinical pregnancy (RR 0.93, 95% CI 0.40 to 2.13; 1 RCT; 130 participants) and multiple pregnancy rates (RR 1.03, 95% CI 0.22 to 4.92; 1 RCT; 130 participants) is very uncertain. 6. Luteal support in natural cycle No study evaluated the effect of LPS in natural cycle. We could not perform sensitivity analyses, as there were no studies at low risk of selection bias and not at high risk in other domains.
Authors' conclusions: We are uncertain if vaginal progesterone supplementation during luteal phase is associated with a higher live birth/ongoing pregnancy rate. Vaginal progesterone may increase clinical pregnancy rate; however, its effect on miscarriage rate and multiple pregnancy rate is uncertain. We are uncertain if IM progesterone improves ongoing pregnancy rates or decreases miscarriage rate when compared to vaginal progesterone. Regarding the other reported comparisons, neither oral progesterone nor any other medication appears to be associated with an improvement in pregnancy outcomes (very low-certainty evidence).
Copyright © 2022 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
LS declares no conflict of interest.
DMT declares no conflict of interest.
IS declares no conflict of interest.
JS declares no conflict of interest.
WPM declares no conflict of interest.
MBR declares no conflict of interest.
PL declares no conflict of interest.
Figures
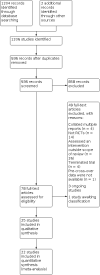































Update of
- doi: 10.1002/14651858.CD012396
Similar articles
-
Gonadotropins for ovulation induction in women with polycystic ovary syndrome.Cochrane Database Syst Rev. 2025 Apr 7;4(4):CD010290. doi: 10.1002/14651858.CD010290.pub4. Cochrane Database Syst Rev. 2025. PMID: 40193219
-
Luteal phase support for assisted reproduction cycles.Cochrane Database Syst Rev. 2015 Jul 7;2015(7):CD009154. doi: 10.1002/14651858.CD009154.pub3. Cochrane Database Syst Rev. 2015. PMID: 26148507 Free PMC article.
-
Endometrial injury for pregnancy following sexual intercourse or intrauterine insemination.Cochrane Database Syst Rev. 2022 Oct 24;10(10):CD011424. doi: 10.1002/14651858.CD011424.pub4. Cochrane Database Syst Rev. 2022. PMID: 36278845 Free PMC article.
-
Endometrial injury for pregnancy following sexual intercourse or intrauterine insemination.Cochrane Database Syst Rev. 2016 Jun 14;(6):CD011424. doi: 10.1002/14651858.CD011424.pub2. Cochrane Database Syst Rev. 2016. Update in: Cochrane Database Syst Rev. 2021 Mar 18;3:CD011424. doi: 10.1002/14651858.CD011424.pub3. PMID: 27296541 Updated.
-
Cycle regimens for frozen-thawed embryo transfer.Cochrane Database Syst Rev. 2017 Jul 5;7(7):CD003414. doi: 10.1002/14651858.CD003414.pub3. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2025 Jun 3;6:CD003414. doi: 10.1002/14651858.CD003414.pub4. PMID: 28675921 Free PMC article. Updated.
Cited by
-
Progesterone: The Key Factor of the Beginning of Life.Int J Mol Sci. 2022 Nov 16;23(22):14138. doi: 10.3390/ijms232214138. Int J Mol Sci. 2022. PMID: 36430614 Free PMC article. Review.
-
Optimizing intrauterine insemination: A systematic review and meta-analysis of the effectiveness and safety of clinical treatment add-ons.Acta Obstet Gynecol Scand. 2024 Oct;103(10):1919-1932. doi: 10.1111/aogs.14858. Epub 2024 Jul 3. Acta Obstet Gynecol Scand. 2024. PMID: 38961556 Free PMC article.
-
Assisted reproductive technology: an overview of Cochrane Reviews.Cochrane Database Syst Rev. 2018 Aug 17;8(8):CD010537. doi: 10.1002/14651858.CD010537.pub5. Cochrane Database Syst Rev. 2018. PMID: 30117155 Free PMC article.
References
References to studies included in this review
Aali 2013 {published data only}
Agha‐Hosseini 2012 {published data only}
-
- Agha-Hosseini M, Rahmani M, Alleyassin A, Safdarian L, Sarvi F. The effect of progesterone supplementation on pregnancy rates in controlled ovarian stimulation and intrauterine insemination cycles: a randomised prospective trial. European Journal of Obstetrics & Gynecology and Reproductive Biology 2012;165(2):249-53. - PubMed
-
- Alleyassin A, Agha Hosseini M, Sarvi F, Safdarian L, Rahmanpour H, Kokab AA. The effect of progesterone supplementation on pregnancy rates in controlled ovarian stimulation and intrauterine insemination cycles: a randomized prospective trial. Fertility and Sterility 2012;98(3 Suppl 1):S197. - PubMed
Alizzi 2018 {published data only}
-
- Alizzi FJ. Pregnancy rate following luteal phase support in polycystic ovary women using letrozole with or without gonadotropin as ovulation induction. Asian Journal of Pharmacuetical and Clinical Research 2018;11(9):321-4.
An 2010 {published data only}
-
- An SJ, Jung JY, Kwon HC, Kim HM, Han JH, Lee SJ. Effect of luteal phase support in intrauterine insemination cycles: a prospective randomised study. Fertility and Sterility 2010;94 Suppl 1(4):S176-7.
Azmoodeh 2016 {published data only}
-
- Azmoodeh A, Mohamadpoor J, Asbagh FA, Ghaseminezhad A, Lorzadeh N, Forghani F. Comparision the effect of GNRH agonist administration versus vaginal progesterone on serum progesterone in luteal phase in ovarian hyperstimulation and intrauterine insemination cycles in unexplained infertility. International Journal of Medicine and Health Research 2016;2(9):84-8.
-
- Mohamadpoor J. Comparision the effect of GNRH agonist administration versus vaginal progesterone on serum progesterone in luteal phase in ovarian hyperstimulation and intrauterine insemination cycles in unexplained infertility. International Journal of Fertility and Sterility 2013;7:112.
Bekuretsion 1998 {published data only}
-
- Bekuretsion M, Lundkvist O, Karlstrom PO. A randomised study comparing low and high dose of HMG/FSH with and without luteal phase support in intrauterine insemination cycles. Human Reproduction 1998;13:199.
Bellver 2010 {published data only}
-
- Bellver J, Labarta E, Bosch E, Melo MA, Vidal C, Remohi J, et al. GnRH agonist administration at the time of implantation does not improve pregnancy outcomes in intrauterine insemination cycles: a randomised controlled trial. Fertility and Sterility 2010;94:1065-71. - PubMed
Biberoglu 2016 {published data only}
-
- Biberoglu EH, Tanrikulu F, Erdem M, Erdem A, Biberoglu KO. Luteal phase support in intrauterine insemination cycles: a prospective randomised study of 300 mg versus 600 mg intravaginal progesterone tablet. Gynecological Endocrinology 2016;32(1):55-7. - PubMed
Busso 2006 {published data only}
-
- Busso C, Labarta E, Melo MAB, Navarro C, Vidal C, Remohi J, et al. Effect of a single dose of a GnRH agonist in the luteal phase of intrauterine insemination cycles: a randomised study. Interim analysis. Human Reproduction 2006;21(Suppl 1):i116.
Eguiluz 2012 {published data only}
-
- Eguiluz Gutierrez-Barquin I, Sanchez Sanchez V, Torres Afonso A, Alvarez Sanchez M, De Leon Socorro S, Molina Cabrillana J, et al. Should luteal phase support be introduced in ovarian stimulation/IUI programme? Human Reproduction 2012;27:ii226. [Abstract number: P320]
-
- Eguiluz Gutierrez-Barquin I, Sanchez Sanchez V, Torres Afonso A, Alvarez Sanchez M, De Leon Socorro S, Molina Cabrillana J, et al. Should luteal phase support be introduced in ovarian stimulation/IUI programme? Human Reproduction 2012;27 Suppl 2(12):ii226-47.
Erdem 2009 {published data only}
-
- Erdem A, Erdem M, Atmaca S, Guler I. Impact of luteal phase support on pregnancy rates in intrauterine insemination cycles: a prospective randomised study. Fertility and Sterility 2009;91:2508-13. - PubMed
Han 2016 {published data only}
-
- Han J, Motan T. The effect of luteal phase support on pregnancy rates in intrauterine insemination cycles following ovarian stimulation with gonadotropins - a randomised controlled trial. Fertility and Sterility 2016;106(3):e57.
Karadag 2016 {published data only}
-
- Karadag B, Dilbaz B, Karcaaltincaba D, Sahin EG, Ercan F, Karasu Y, et al. The effect of luteal-phase support with vaginal progesterone on pregnancy rates in gonadotropin and clomiphene citrate/intra-uterine insemination cycles in unexplained infertility: a prospective randomised study. Journal of Obstetrics and Gynaecology 2016;36:794-9. - PubMed
Keskin 2020 {published data only}
Khadem 2011 {published data only}
-
- Khadem N, Mousavifar N, Bonakdar F, Rafie NB. A comparative study of intramuscular oil progesterone injection and suppository progesterone for luteal phase support in patients undergoing IUI cycles. Iranian Journal of Obstetrics, Gynecology and Infertility 2011;13(6):1-5.
-
- Khadem N, Mousavifar N, Bonakdar F, Rafie NB. A comparative study of intramuscular oil progesterone injection and suppository progesterone for luteal phase support in patients undergoing IUI cycles. Iranian Journal of Obstetrics, Gynecology and Infertility 2011;13(6):1-5.
Khosravi 2015 {published data only}
-
- Khosravi D, Taheripanah R, Khosravi R, Taheri Panah A, Tarighat Monfared V, Defaee S, et al. Comparison of oral dydrogesterone with vaginal progesterone for luteal support in IUI cycles: a randomised clinical trial (RCT). Iranian Journal of Obstetrics, Gynecology and Infertility 2015;13:433-8. - PMC - PubMed
Kyrou 2010 {published data only}
-
- Kyrou D, Fatemi HM, Devroey P. The effect of luteal support on pregnancy rates in normo-ovulatory patients stimulated with clomiphene citrate for IUI: a prospective randomised study. Human Reproduction 2010;25 Suppl 1(6):i44-5. [Abstract no. O] - PubMed
-
- Kyrou D, Fatemi HM, Tournaye H, Devroey P. Luteal phase support in normo-ovulatory women stimulated with clomiphene citrate for intrauterine insemination: need or habit? Human Reproduction 2010;25:2501-6. - PubMed
Pakrashi 2014 {published data only}
-
- Pakrashi T, Baydoun H, Bocca S, Oehninger S, Stadtmauer L. Luteal phase supplementation with vaginal progesterone in women with polycystic ovary syndrome and ovulatory dysfunction undergoing ovulation induction with letrozole: a randomised controlled trial. Fertility and Sterility 2014;102 Suppl(3):e237.
Peeraer 2016 {published data only}
-
- Peeraer K, D'Hooghe T, Laurent P, Pelckmans S, Delvigne A, Laenen A, et al. Impact of luteal phase support with vaginal progesterone on the clinical pregnancy rate in intrauterine insemination cycles stimulated with gonadotropins: a randomised multicenter study. Fertility and Sterility 2016;106(6):1490-5. - PubMed
-
- Peeraer K, D'Hooghe T, Laurent P, Pelckmans S, Delvigne A, Laenen A, et al. Impact of luteal phase support with vaginal progesterone on the clinical pregnancy rate in IUI cycles stimulated with gonadotrophins: a prospective randomised multicentre study. Fertility and Sterility 2016;106(6):1490-5. - PubMed
Rashidi 2014 {published data only}
-
- Rashidi BH, Tanha FD, Rahmanpour H, Ghazizadeh M. Luteal phase support in intrauterine insemination (IUI) cycles: a randomised double blind, placebo controlled study [The effect of luteal phase support with vaginal progesterone on pregnancy rates in intrauterine insemination (IUI) cycles]. Fertility and Sterility 2014;8:149-53. - PMC - PubMed
Romero Nieto 2014 {published data only}
-
- Romero Nieto MI, Lorente Gonzalez J, Arjona-Berral JE, Del Munoz-Villanueva M, Castelo-Branco C. Luteal phase support with progesterone in intrauterine insemination: a prospective randomised study. Gynecological Endocrinology 2014;30(3):197-201. - PubMed
Seckin 2014 {published data only}
-
- Seckin B, Turkcapar F, Yildiz Y, Senturk B, Yilmaz N, Gulerman C. Effect of luteal phase support with vaginal progesterone in intrauterine insemination cycles with regard to follicular response: a prospective randomised study. Journal of Reproductive Medicine 2014;59(5):260-6. - PubMed
Stadtmauer 2015 {published data only}
-
- Stadtmauer L, Pakrashi T, Beydoun H, Bocca S, Oehninger S. A randomised controlled trial of luteal phase supplementation with vaginal progesterone in women with polycystic ovary syndrome undergoing ovulation induction with letrozole. Human Reproduction 2015;30 Suppl 1(P-718):i429. [Abstract no: P-718]
-
- Stadtmauer L, Pakrashi T, Beydoun H, Bocca S, Oehninger S. A randomised controlled trial of luteal phase supplementation with vaginal progesterone in women with polycystic ovary syndrome undergoing ovulation induction with letrozole. Human Reproduction 2015;30 Suppl 1(P-718):i429.
Venturella 2016 {published data only}
-
- Venturella R, Mocciaro R, Lico D, Rocca M, Di Cello A, Piccione F, et al. Subcutaneous aqueous versus vaginal progesterone gel for luteal phase support in intrauterine insemination cycles: a pilot randomised, controlled trial. Human Reproduction 2016;31 Suppl 1:i304-5.
Yazici 2014 {published data only}
-
- Yazici G, Savas A, Tasdelen B, Dilek S. Role of luteal phase support on gonadotropin ovulation induction cycles in patients with polycystic ovary syndrome. Journal of Reproductive Medicine 2014;59(1-2):25-30. - PubMed
References to studies excluded from this review
Aboulghar 2009 {published data only}
-
- Aboulghar M. Luteal support in reproduction: when, what and how? Current Opinion in Obstetrics and Gynecology 2009;21(3):279-84. - PubMed
Arango 1997 {published data only}
-
- Arango MartÃnez A, Betancourt MarÃn J. Utilidad del soporte en fase lutea con óvulos de progesterone in artificial intrauterine insemination. Profamilia-in ser. 1996-1997. CES Medicina 1997;11(1):1-25.
Atmaca 2007 {published data only}
-
- Atmaca S, Erdem M, Erdem A, Guler I. The impact of luteal phase support on pregnancy rates in intrauterine insemination cycles: a prospective randomised study. Fertility and Sterility 2007;88 Suppl 1:163. - PubMed
Bachus 1990 {published data only}
-
- Bachus KE, Hughes CL Jr, Haney AF, Dodson WC. The luteal phase in polycystic ovary syndrome during ovulation induction with human menopausal gonadotropin with human menopausal gonadotropin with and without leuprolide acetate. Fertility and Sterility 1990;54(1):27-31. - PubMed
Bakay 2015 {published data only}
-
- Bakay K, Aytekin F, Celik NY. Progesterone supplement and luteal phase deficiency in uni-follicular intrauterine insemination cycles. Journal of Clinical and Analytical Medicine 2015;6:691-3.
Balasch 1983 {published data only}
-
- Balasch J, Vanrell JA, Márquez M, González-Merlo J. Dehydrogesterone treatment of endometrial luteal phase deficiency after ovulation induced by clomiphene citrate and human chorionic gonadotropin. Fertility and Sterility 1983;40(4):469-71. - PubMed
Beltsos 2014 {published data only}
Blumenfeld 1988 {published data only}
-
- Blumenfeld Z, Nahhas F. Luteal dysfunction in ovulation induction: the role of repetitive human chorionic gonadotropin supplementation during the luteal phase. Fertility and Sterility 1988;50(3):403-7. - PubMed
Check 1989 {published data only}
-
- Check JH, Wu CH, Adelson HG. Bromocriptine versus progesterone therapy for infertility related to luteal phase defects in hyperprolactinaemia patients. International Journal of Fertility 1989;34(3):209-14. - PubMed
Cohlen 2009a {published data only}
-
- Cohlen BJ. Should luteal phase support be introduced in ovarian stimulation/IUI programmes? An evidence-based review. Reproductive Biomedicine Online 2009;19(Suppl 4):31-8. - PubMed
Corson 1993 {published data only}
-
- Corson SL, Batzer FR, Gocial B, Maislin G. The luteal phase after ovulation induction with human menopausal gonadotropin and one versus two doses of a gonadotropin-releasing hormone agonist. Fertility and Sterility 1993;59(6):1251-6. - PubMed
Duffy 2006 {published data only}
-
- Duffy DA, Manzi D, Benadiva C, Maier D, Saunders M, Nulsen J. Impact of leuprolide acetate on luteal phase function in women undergoing controlled ovarian hyperstimulation and intrauterine insemination. Fertility and Sterility 2006;85(2):407-11. - PubMed
Elkind 2000 {published data only}
-
- Elkind Hirsch K, Phillips K, Keller MG, Bello S, McNichol M. Hormone supplementation (vaginal oestradiol and Crinone 8%) corrects delayed endometrial development during the late luteal phase. Human Reproduction 2000;15:174.
Ergur 1998 {published data only}
-
- Ergur AR, Yergok Y, Ertekin A, Mungen E, Tutuncu L. Efficacy of luteal phase supplementation with human chorionic gonadotropin (HCG) in gonadotropin induced cycles. Human Reproduction 1997;12(Suppl 2):322-3.
Foroozanfard 2012 {published data only}
-
- Foroozanfard F, Saberi H, Moravvegi A, Kazemi M. Vaginal progesterone effect on pregnancy rate in polycystic ovary syndrome patients. Iranian Journal of Reproductive Medicine 2012;10:10.
Foroozanfard 2013 {published data only}
-
- Foroozanfard F, Saberi H, Moravvegi A, Kazemi M. Pregnancy rate following luteal phase support in polycystic ovarian syndrome using combination therapies for ovulation induction: a randomised clinical trial. Human Reproduction 2013;28:i311-56 P.
-
- Foroozanfard F, Saberi H, Moravvegi A, Kazemi M. Pregnancy rate following luteal phase support in polycystic ovarian syndrome using combination therapies for ovulation induction: a randomised clinical trial. Human Reproduction 2013;28 Suppl 1:i311-56 P.
Gagliardi 1993 {published data only}
-
- Gagliardi CL. Utility of gonadotropin-releasing hormone agonists in programs of ovarian hyperstimulation with intrauterine insemination. Clinical Obstetrics & Gynecology 1993;36(3):711-8. - PubMed
Gleicher 2000 {published data only}
-
- Gleicher N, Brown T, Dudkiewicz A, Karande V, Rao R, Balin M, et al. Estradiol/progesterone substitution in the luteal phase improves pregnancy rates in stimulated cycles - but only in younger women. Early Pregnancy: Biology and Medicine 2000;4(1):64-73. - PubMed
Green 2017a {published data only}
-
- Green KA, Zolton JR, Schermerhorn SM, DeCherney AH, Hill MJ. An updated meta-analysis evaluating the effect of progesterone (P) luteal support on live birth after ovulation induction (OI) and intrauterine insemination (IUI). Reproductive Sciences 2017;1(Suppl 1):166A.
Gun 2016 {published data only}
Hansen 2018 {published data only}
IRCT201202078948N1 {published data only}
-
- IRCT201202078948N1. The effect of progesterone in intrauterine insemination [Comparison the efficacy of using intravaginal progesterone in pregnancy rate in intrauterine insemination]. www.irct.ir/trial/9407 (first received 17 August 2012).
IRCT2015030521344N1 {published data only}
-
- IRCT2015030521344N1. Comparing intramuscular (25 and 50 milligram) and vaginal suppository (400 and 800 milligram) progesterone for luteal phase support in IUI cycle in infertile women with age of 20 to 38 years, who were candidate for IUI cycles due to male factor and unexplained infertility [Comparison of intramuscular progesterone and suppository progesterone for luteal phase support in IUI cycles]. www.irct.ir/trial/18732 (first received 1 May 2015).
Keenan 1992 {published data only}
-
- Keenan JA, Moghissi KS. Luteal phase support with hCG does not improve fecundity rate in human menopausal gonadotropin-stimulated cycles. Obstetrics and Gynecology 1992;79(6):983-7. - PubMed
Lebrocquy 1998 {published data only}
-
- Lebrocquy C, Geriil A, Janssen J, Evenepoel J, Branden K. Results of an artificial insemination programme with cryopreserved donor spermatozoa with or without luteal supplementation in cycles with clomiphene citrate. Human Reproduction 1998;13(1):19.
Ludwig 2001 {published data only}
-
- Ludwig M, Finas A, Katalinic A, Strik D, Kowalcek I, Schwartz P, et al. Prospective, randomised study to evaluate the pregnancy rate using HCG, vaginal progesterone (Utrogest), or a combination of both for luteal-phase support: preliminary results. Acta Obstetricia et Gynecologica Scandinavica 2001;80(1):574-82. - PubMed
Madkour 2016 {unpublished data only}
-
- Madkour WA, Noah B, Arm MS, Hamid A, Zeheer H, Al-Bahr A, et al. Luteal phase support with progesterone versus estrogen and progesterone on pregnancy rates. Human Fertility 2016;19:142-9. - PubMed
Maher 2011 {published data only}
-
- Maher MA. Luteal phase support may improve pregnancy outcomes during intrauterine insemination cycles. European Journal of Obstetrics & Gynecology and Reproductive Biology 2011;157:57-62. - PubMed
Malhotra 2016 {published data only}
Malik 2016 {published data only}
Martins 2010 {published data only}
-
- Martins WP. Suporte da fase lútea. Femina 2010;38(5):271-6.
Miralpeix 2014a {published data only}
Miralpeix 2016 {published data only}
Mukherjee 2016 {published data only}
-
- Mukherjee G, Konar H, Mandve P, Sharma S, Ghosh S, Chakraborty P, et al. Progesterone supplementation in PCOS women undergoing clomiphene citrate stimulated IUI may improve pregnancy by increasing uterine blood. Fertility and Sterility 2016;106:e260.
NCT00700492 (a) {published data only}
-
- NCT00700492. Does hormonal luteal support after intra-uterine insemination (IUI) increase the pregnancy rate? www.clinicaltrials.gov/ct2/show/NCT00700492 (first received 18 June 2008).
NCT00700492 (b) {published data only}
-
- NCT00700492. Does hormonal luteal support after intra-uterine insemination (IUI) increase the pregnancy rate? clinicaltrials.gov/ct2/show/NCT00700492 (first received 18 June 2008).
NCT02510534 (a) {published data only}
-
- NCT02510534. Effect of luteal progesterone support on pregnancy rates with combined clomid/ gonadotropin & IUI. clinicaltrials.gov/ct2/show/NCT02510534 (first received 23 September 2016).
NCT02510534 (b) {published data only}
-
- NCT02510534. Effect of luteal progesterone support on pregnancy rates with combined clomid/gonadotropin & IUI. clinicaltrials.gov/ct2/show/NCT02510534 (first received 29 July 2015).
Niles 2019 {published data only}
-
- Niles AM, Fricke HP, Carvalho PD, Wiltbank MC, Hernandez LL, Fricke PM. Effect of treatment with human chorionic gonadotropin 7 days after artificial insemination or at the time of embryo transfer on reproductive outcomes in nulliparous Holstein heifers. Journal of Dairy Science 2019;102(3):2593-606. - PubMed
Ozcimen 2004 {published data only}
-
- Ozcimen E, Ugur M, Ozcimen N, Yilmaz Z. Is luteal phase support with hCG or vaginal micronized progesterone beneficial in non-IVF gonadotropin induction of ovulation? Fertility and Sterility 2004;82(Suppl 2):S142.
Penarrubia 1998 {published data only}
-
- Peñarrubia J, Balasch J, Fábregues F, Creus M, Casamitjana R, Ballescá JL, et al. Human chorionic gonadotrophin luteal support overcomes luteal phase inadequacy after gonadotrophin-releasing hormone agonist-induced ovulation in gonadotrophin-stimulated cycles. Human Reproduction 1998;13(12):3315-8. - PubMed
Pirard 2005 {published data only}
-
- Pirard C, Donnez J, Loumaye E. GnRH agonist as novel luteal support: results of a randomised, parallel group, feasibility study using intranasal administration of buserelin. Human Reproduction 2005;20(7):1798-804. - PubMed
Pomettini 2001 {published data only}
-
- Pomettini A, Capalbi A, Melluso J, Reale MG, Agostini R. Progesterone supplementation for induction of ovulation. Giomale Italiano di Ostetrica e Gonecologia 2001;23(6):285-9.
Schwarze 2013 {published data only}
-
- Schwarze JE, Villa S, Manzur A, Magendzo A, Pommer R. Progesterone-releasing vaginal ring for luteal phase support after superovulation and intrauterine insemination cycles, a pilot study. JBRA Assisted Reproduction 2013;17:313-5.
Tas 2020 {published data only}
-
- Taş M, Uludag SZ, Aygen ME, Sahin Y. Comparison of oral dydrogesterone and vaginal micronized progesterone for luteal phase support in intrauterine insemination. Gynecology Endocrinology 2020;36(1):77-80. - PubMed
-
- Taş M, Uludag SZ, Aygen ME, Sahin Y. Comparison of oral dydrogesterone and vaginal micronized progesterone for luteal phase support in intrauterine insemination. Gynecology Endocrinology 2020;36(1):77-80. - PubMed
Yilmaz 2006 {published data only}
-
- Yilmaz B, Kelekci S, Savan K, Oral H, Mollamahmutoglu L. Addition of human chorionic gonadotropin to clomiphene citrate ovulation induction therapy does not improve pregnancy outcomes and luteal function. Fertility and Sterility 2006;85(3):783-6. - PubMed
Youssef 2000 {published data only}
-
- Youssef G, Maguid A, El-Inany H. Progesterone supplementation in clomiphene citrate treated anovulatory patients with menstrual irregularities: a randomised controlled trial. Middle East Fertility Society Journal 2000;5(3):209-12.
Zaffaroni 1997 {published data only}
-
- Zaffaroni L, Costa P, Vinci G. Topical progesterone as support for the luteal phase in induced cycles. Minerva Gynaecology 1997;49:361-4. - PubMed
Zayed 2003 {published data only}
-
- Zayed FF, El-Jallad MF, Al-Chalabi HA. Luteal phase support in ovarian induction cycles using human chorionic gonadotropin or oral progestogens. Saudi Medical Journal 2003;24(1):34-6. - PubMed
References to studies awaiting assessment
Ebrahimi 2010 {published data only}
-
- Ebrahimi M, Asbagh FA, Darvish S. The effect of luteal phase support on pregnancy rates of the stimulated intrauterine insemination cycles in couples with unexplained infertility. International Journal of Fertility and Sterility 2010;4:51-6.
References to ongoing studies
Maryam 2017 {published data only}
-
- IRCT2017041233379N. The effect of progesterone on pregnancy rates in the intrauterine insemination cycles [The effect of progesterone on pregnancy rates in the intrauterine insemination cycles]. apps.who.int/trialsearch/Trial2.aspx?TrialID=IRCT2017041233379N1 (first received 22 February 2018).
-
- IRCT2017041233379N1. The effect of progesterone on pregnancy rates in the intrauterine insemination cycles [The effect of progesterone suppository to luteal phase support on pregnancy rates in the intrauterine insemination cycles]. apps.who.int/trialsearch/Trial2.aspx?TrialID=IRCT2017041233379N1 (first received 22 February 2018).
NCT03115307 {published data only}
-
- NCT03115307. Luteal phase support in insemination cycles [A protocol for a randomized, controlled study to compare the use of gonodotropin-releasing hormone agonist triptoreline (Gonapeptyl®) for luteal phase support versus natural luteal phase in the insemination cycles]. clinicaltrials.gov/ct2/show/NCT03115307 (first received 14 April 2017).
NCT03440359 {published data only}
-
- NCT03440359. Vaginal progesterone supplementation in women with PCOS undergoing ovulation induction with letrozole [Supplementation of the luteal phase with vaginal progesterone (Crinone 8%) in women with polycystic ovary syndrome undergoing ovulation induction with letrozole: a prospective and randomized controlled trial]. clinicaltrials.gov/ct2/show/NCT03440359 (first received 22 Febuary 2018).
Additional references
Aboulghar 2015
Adamson 2003
-
- Adamson GD, Baker VL. Subfertility: causes, treatment and outcome. Best Practice & Research. Clinical Obstetrics & Gynaecology 2003;17(2):169-85. [PMID: ] - PubMed
Andersen 2015
-
- Andersen CY, Elbaek HO, Alsbjerg B, Laursen RJ, Povlsen BB, Thomsen L, et al. Daily low-dose hCG stimulation during the luteal phase combined with GnRHa triggered IVF cycles without exogenous progesterone: a proof of concept trial. Human Reproduction 2015;30(10):2387-95. [DOI: 10.1093/humrep/dev184] [PMID: ] - DOI - PubMed
Andrews 2015
Arredondo 2006
-
- Arredondo F, Noble LS. Endocrinology of recurrent pregnancy loss. Seminars in Reproductive Medicine 2006;24(1):33-9. [PMID: ] - PubMed
Barbosa 2016
-
- Barbosa MW, Silva LR, Navarro PA, Ferriani RA, Nastri CO, Martins WP. Dydrogesterone vs progesterone for luteal-phase support: systematic review and meta-analysis of randomised controlled trials. Ultrasound in Obstetrics and Gynecology 2016;48(2):161-70. [DOI: 10.1002/uog.15814] [PMID: ] - DOI - PubMed
Birch Petersen 2016
Blake 2010
-
- Blake EJ, Norris PM, Dorfman SF, Longstreth J, Yankov VI. Single and multidose pharmacokinetic study of a vaginal micronized progesterone insert (Endometrin) compared with vaginal gel in healthy reproductive-aged female subjects. Fertility and Sterility 2010;94(4):1296-301. [DOI: 10.1016/j.fertnstert.2009.06.014] [PMID: ] - DOI - PubMed
Boivin 2007
Boutzios 2013
Bukman 2000
-
- Bukman A, Heineman MJ. Ovarian reserve testing and the use of prognostic models in patients with subfertility. Human Reproduction Update 2001;7(6):581-90. - PubMed
Bulun 2010
Chwalisz 2000
-
- Chwalisz K, Garfield RE. Role of nitric oxide in implantation and menstruation. Human Reproduction 2000;15(Suppl 3):96-111. [PMID: ] - PubMed
Cohlen 2009b
-
- Cohlen BJ. Should luteal phase support be introduced in ovarian stimulation/IUI programmes? An evidence-based review. Reproductive Biomedicine Online 2009;19(Suppl 4):4239-46. [PMID: 20034415] - PubMed
Covidence [Computer program]
-
- Covidence. Version accessed 7 November 2019. Melbourne, Australia: Veritas Health Innovation. Available at covidence.org.
Druckmann 2005
-
- Druckmann R, Druckmann MA. Progesterone and the immunology of pregnancy. Journal of Steroid Biochemistry and Molecular Biology 2005;97(5):389-96. [PMID: ] - PubMed
Fanchin 1998
-
- Fanchin R, Righini C, Olivennes F, Taylor S, Ziegler D, Frydman R. Uterine contractions at the time of embryo transfer alter pregnancy rates after in-vitro fertilization. Human Reproduction 1998;13(7):1968-74. [PMID: 9740459] - PubMed
Franik 2018
Gnoth 2005
GRADEpro GDT [Computer program]
-
- GRADEpro GDT. Version accessed 11 May 2020. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Green 2017b
-
- Green KA, Zolton JR, Schermerhorn SM, Lewis TD, Healy MW, Terry N, et al. Progesterone luteal support after ovulation induction and intrauterine insemination: an updated systematic review and meta-analysis. Fertility and Sterility 2017;107:924-33. [DOI: 10.1016/j.fertnstert.2017.01.011] [PMID: ] - DOI - PubMed
Haouzi 2014
Higgins 2021
-
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2.
Kim 2015
Lensen 2021
Levy 2000
-
- Levy T, Yairi Y, Bar-Hava I, Shalev J, Orvieto R, Ben-Rafael Z. Pharmacokinetics of the progesterone-containing vaginal tablet and its use in assisted reproduction. Steroids 2000;65(10-1):645-9. - PubMed
Martins 2013
-
- Martins WP, Vieira AD, Figueiredo JB, Nastri CO. FSH replaced by low-dose hCG in the late follicular phase versus continued FSH for assisted reproductive techniques. Cochrane Database of Systematic Reviews 2013, Issue 3. Art. No: CD010042. [DOI: 10.1002/14651858.CD010042.pub2] [PMID: ] - DOI - PMC - PubMed
Martins 2015
-
- Martins WP, Ferriani RA, Navarro PA, Nastri CO. GnRH agonist during luteal phase for women undergoing assisted reproductive techniques: systematic review and meta-analysis of randomised controlled trials. Ultrasound in Obstetrics and Gynecology 2015;47(2):144-51. [DOI: 10.1002/uog.14874] [PMID: ] - DOI - PubMed
Miralpeix 2014b
Nastri 2015
-
- Nastri CO, Teixeira DM, Moroni RM, Leitão VM, Martins WP. Ovarian hyperstimulation syndrome: pathophysiology, staging, prediction and prevention. Ultrasound in Obstetrics and Gynecology 2015;45(4):377-93. - PubMed
Navot 1992
-
- Navot D, Bergh PA, Laufer N. Ovarian hyperstimulation syndrome in novel reproductive technologies: prevention and treatment. Fertility and Sterility 1992;58(2):249-61. [PMID: ] - PubMed
Nillius 1971
-
- Nillius SJ, Johansson ED. Plasma levels of progesterone after vaginal, rectal, or intramuscular administration of progesterone. American Journal of Obstetrics and Gynecology 1971;110(4):470-7. [PMID: ] - PubMed
Review Manager 2020 [Computer program]
-
- Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Sator 2013
-
- Sator M, Radicioni M, Cometti B, Loprete L, Leuratti C, Schmidl D, et al. Pharmacokinetics and safety profile of a novel progesterone aqueous formulation administered by the subcutaneous route. Gynecological Endocrinology 2013;29(3):205-8. - PubMed
Shah 2013
Shaw 2010
Simon 1993
-
- Simon JA, Robinson DE, Andrews MC, Hildebrand JR 3rd, Rocci ML Jr, Blake RE, et al. The absorption of oral micronized progesterone: the effect of food, dose proportionality, and comparison with intramuscular progesterone. Fertility and Sterility 1993;60(1):26-33. [PMID: ] - PubMed
van der Linden 2015
References to other published versions of this review
Checa 2016
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

