Single-molecule analysis of the entire perfringolysin O pore formation pathway
- PMID: 36000711
- PMCID: PMC9457685
- DOI: 10.7554/eLife.74901
Single-molecule analysis of the entire perfringolysin O pore formation pathway
Abstract
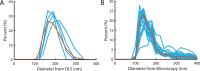
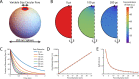
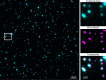
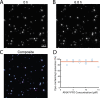
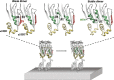
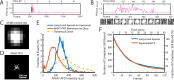
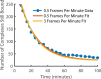
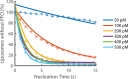
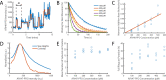
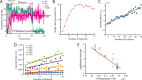
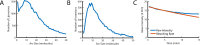
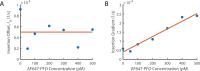
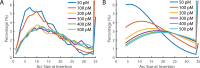
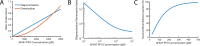
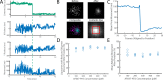
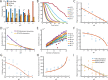
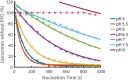
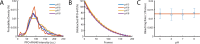
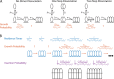
The cholesterol-dependent cytolysin perfringolysin O (PFO) is secreted by Clostridium perfringens as a bacterial virulence factor able to form giant ring-shaped pores that perforate and ultimately lyse mammalian cell membranes. To resolve the kinetics of all steps in the assembly pathway, we have used single-molecule fluorescence imaging to follow the dynamics of PFO on dye-loaded liposomes that lead to opening of a pore and release of the encapsulated dye. Formation of a long-lived membrane-bound PFO dimer nucleates the growth of an irreversible oligomer. The growing oligomer can insert into the membrane and open a pore at stoichiometries ranging from tetramers to full rings (~35 mers), whereby the rate of insertion increases linearly with the number of subunits. Oligomers that insert before the ring is complete continue to grow by monomer addition post insertion. Overall, our observations suggest that PFO membrane insertion is kinetically controlled.
Keywords: assembly kinetics; biochemistry; chemical biology; cholesterol dependent cytolysin; membrane pore; molecular biophysics; none; single-molecule fluorescence; structural biology.
© 2022, Mc Guinness, Walsh et al.
Conflict of interest statement
CM, JW, CB, MD, MC, CM, MP, TB No competing interests declared
Figures











Similar articles
-
Single-molecule tracking of perfringolysin O assembly and membrane insertion uncoupling.FEBS J. 2023 Jan;290(2):428-441. doi: 10.1111/febs.16596. Epub 2022 Sep 19. FEBS J. 2023. PMID: 35989549 Free PMC article.
-
Perfringolysin O: The Underrated Clostridium perfringens Toxin?Toxins (Basel). 2015 May 14;7(5):1702-21. doi: 10.3390/toxins7051702. Toxins (Basel). 2015. PMID: 26008232 Free PMC article. Review.
-
Phospholipid hydrolysis caused by Clostridium perfringens α-toxin facilitates the targeting of perfringolysin O to membrane bilayers.Biochemistry. 2010 Nov 9;49(44):9498-507. doi: 10.1021/bi1013886. Biochemistry. 2010. PMID: 20886855
-
R468A mutation in perfringolysin O destabilizes toxin structure and induces membrane fusion.Biochim Biophys Acta Biomembr. 2017 Jun;1859(6):1075-1088. doi: 10.1016/j.bbamem.2017.03.001. Epub 2017 Mar 2. Biochim Biophys Acta Biomembr. 2017. PMID: 28263714
-
Interaction of Cholesterol with Perfringolysin O: What Have We Learned from Functional Analysis?Toxins (Basel). 2017 Nov 23;9(12):381. doi: 10.3390/toxins9120381. Toxins (Basel). 2017. PMID: 29168745 Free PMC article. Review.
Cited by
-
Hexameric-Based Hierarchy in the Sizes of a Cytolysin Pore-Forming Complex.Biomolecules. 2025 Mar 17;15(3):424. doi: 10.3390/biom15030424. Biomolecules. 2025. PMID: 40149960 Free PMC article.
-
The Barrier Disruption and Pyroptosis of Intestinal Epithelial Cells Caused by Perfringolysin O (PFO) from Clostridium perfringens.Cells. 2024 Jul 3;13(13):1140. doi: 10.3390/cells13131140. Cells. 2024. PMID: 38994991 Free PMC article.
-
Dynamic Gene Expression Mitigates Mutational Escape in Lysis-Driven Bacteria Cancer Therapy.Biodes Res. 2024 Sep 19;6:0049. doi: 10.34133/bdr.0049. eCollection 2024. Biodes Res. 2024. PMID: 39301524 Free PMC article.
-
Cholesterol-dependent cytolysins: The outstanding questions.IUBMB Life. 2022 Dec;74(12):1169-1179. doi: 10.1002/iub.2661. Epub 2022 Jul 14. IUBMB Life. 2022. PMID: 35836358 Free PMC article. Review.
-
Pore-Forming Proteins: From Pore Assembly to Structure by Quantitative Single-Molecule Imaging.Int J Mol Sci. 2023 Feb 25;24(5):4528. doi: 10.3390/ijms24054528. Int J Mol Sci. 2023. PMID: 36901959 Free PMC article. Review.
References
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources

