Breast cancer cryoablation with radiologic-pathologic correlation
- PMID: 36000723
- PMCID: PMC9733605
- DOI: 10.1259/bjr.20220480
Breast cancer cryoablation with radiologic-pathologic correlation
Abstract
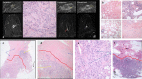
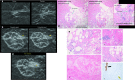
Breast cryoablation has become a viable option for the treatment of breast cancer in properly selected patient populations. With the increase in the use of cryoablation, post-treatment follow-up imaging and pathology has also gained increased importance. By using the proper imaging combination of diagnostic mammography, ultrasound and MRI, physicians are able to detect residual or recurrent malignancy and differentiate it from expected post-treatment findings. If suspicious imaging findings are seen, prompt biopsy and pathological diagnosis are essential. The pathologist must also be able to differentiate the expected post-procedural histological findings from those of recurrent or residual malignancy. These imaging and pathological findings must also be compared in order to ensure concordance and appropriate patient treatment.
Figures


References
-
- McGahan JP, Raalte VA . History of ablation . Tumor Ablation 2005. ; 3 – 16 . doi: 10.1007/0-387-28674-8_1 - DOI
-
- Simmons RM, Ballman KV, Cox C, Carp N, Sabol J, Hwang RF, et al. . A phase II trial exploring the success of cryoablation therapy in the treatment of invasive breast carcinoma: results from ACOSOG (alliance) Z1072 . Ann Surg Oncol 2016. ; 23 : 2438 – 45 . doi: 10.1245/s10434-016-5275-3 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

