Identification and Mutation Analysis of Nonconserved Residues on the TIM-Barrel Surface of GH5_5 Cellulases for Catalytic Efficiency and Stability Improvement
- PMID: 36000858
- PMCID: PMC9469711
- DOI: 10.1128/aem.01046-22
Identification and Mutation Analysis of Nonconserved Residues on the TIM-Barrel Surface of GH5_5 Cellulases for Catalytic Efficiency and Stability Improvement
Abstract
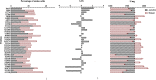
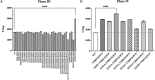
Exploring the potential functions of nonconserved residues on the outer side of α-helices and systematically optimizing them are pivotal for their application in protein engineering. Based on the evolutionary structural conservation analysis of GH5_5 cellulases, a practical molecular improvement strategy was developed. Highly variable sites on the outer side of the α-helices of the GH5_5 cellulase from Aspergillus niger (AnCel5A) were screened, and 14 out of the 34 highly variable sites were confirmed to exert a positive effect on the activity. After the modular combination of the positive mutations, the catalytic efficiency of the mutants was further improved. By using CMC-Na as the substrate, the catalytic efficiency and specific activity of variant AnCel5A_N193A/T300P/D307P were approximately 2.0-fold that of AnCel5A (227 ± 21 versus 451 ± 43 ml/s/mg and 1,726 ± 19 versus 3,472 ± 42 U/mg, respectively). The half-life (t1/2) of variant AnCel5A_N193A/T300P/D307P at 75°C was 2.36 times that of AnCel5A. The role of these sites was successfully validated in other GH5_5 cellulases. Computational analyses revealed that the flexibility of the loop 6-loop 7-loop 8 region was responsible for the increased catalytic performance. This work not only illustrated the important role of rapidly evolving positions on the outer side of the α-helices of GH5_5 cellulases but also revealed new insights into engineering the proteins that nature left as clues for us to find. IMPORTANCE A comprehensive understanding of the residues on the α-helices of the GH5_5 cellulases is important for catalytic efficiency and stability improvement. The main objective of this study was to use the evolutionary conservation and plasticity of the TIM-barrel fold to probe the relationship between nonconserved residues on the outer side of the α-helices and the catalytic efficiency of GH5_5 cellulases by conducting structure-guided protein engineering. By using a four-step nonconserved residue screening strategy, the functional role of nonconserved residues on the outer side of the α-helices was effectively identified, and a variant with superior performance and capability was constructed. Hence, this study proved the effectiveness of this strategy in engineering GH5_5 cellulases and provided a potential competitor for industrial applications. Furthermore, this study sheds new light on engineering TIM-barrel proteins.
Keywords: GH5_5 family cellulases; TIM-barrel fold; catalytic efficiency; lignocellulosic biomass; nonconserved residues; α-helices.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







Similar articles
-
Activity and Thermostability of GH5 Endoglucanase Chimeras from Mesophilic and Thermophilic Parents.Appl Environ Microbiol. 2019 Feb 20;85(5):e02079-18. doi: 10.1128/AEM.02079-18. Print 2019 Mar 1. Appl Environ Microbiol. 2019. PMID: 30552196 Free PMC article.
-
Enhancing the catalytic activity of a novel GH5 cellulase GtCel5 from Gloeophyllum trabeum CBS 900.73 by site-directed mutagenesis on loop 6.Biotechnol Biofuels. 2018 Mar 22;11:76. doi: 10.1186/s13068-018-1080-5. eCollection 2018. Biotechnol Biofuels. 2018. PMID: 29588661 Free PMC article.
-
Functional and structural analysis of Pichia pastoris-expressed Aspergillus niger 1,4-β-endoglucanase.Biochem Biophys Res Commun. 2016 Jun 17;475(1):8-12. doi: 10.1016/j.bbrc.2016.05.012. Epub 2016 May 3. Biochem Biophys Res Commun. 2016. PMID: 27154222
-
Glycosyl hydrolases family 5, subfamily 5: Relevance and structural insights for designing improved biomass degrading cocktails.Int J Biol Macromol. 2021 Dec 15;193(Pt A):980-995. doi: 10.1016/j.ijbiomac.2021.10.062. Epub 2021 Oct 16. Int J Biol Macromol. 2021. PMID: 34666133 Review.
-
β-Glucosidases.Cell Mol Life Sci. 2010 Oct;67(20):3389-405. doi: 10.1007/s00018-010-0399-2. Epub 2010 May 20. Cell Mol Life Sci. 2010. PMID: 20490603 Free PMC article. Review.
Cited by
-
Semi-rational engineering of an α-L-fucosidase for regioselective synthesis of fucosyl-N-acetylglucosamine disaccharides.Food Chem (Oxf). 2025 Feb 11;10:100244. doi: 10.1016/j.fochms.2025.100244. eCollection 2025 Jun. Food Chem (Oxf). 2025. PMID: 40034538 Free PMC article.
References
-
- Bilal M, Wang Z, Cui J, Ferreira LFR, Bharagava RN, Iqbal HMN. 2020. Environmental impact of lignocellulosic wastes and their effective exploitation as smart carriers–a drive towards greener and eco-friendlier biocatalytic systems. Sci Total Environ 722:137903. 10.1016/j.scitotenv.2020.137903. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

