Extracellular matrix regulation of stress response genes during larval development in Caenorhabditis elegans
- PMID: 36000892
- PMCID: PMC9635657
- DOI: 10.1093/g3journal/jkac221
Extracellular matrix regulation of stress response genes during larval development in Caenorhabditis elegans
Abstract
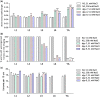
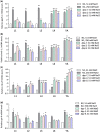
Mutation or loss of 6 extracellular matrix collagen genes disrupts annular furrows in adult C. elegans cuticles, causes a wide "Dumpy" body morphology, and activates osmotic, detoxification, and antimicrobial defense genes. High environmental osmolarity reduces internal turgor pressure, physically distorts the epidermis, and activates the same stress responses. Collagen gene mutations that cause Dumpy without furrow disruption do not activate stress responses. These results are consistent with an extracellular damage sensor associated with furrows in the adult cuticle that regulates environmental stress responses in adjacent cells. Several cuticle characteristics change between molts, but all stages have annular furrows and express furrow collagen genes. We compared body shape, furrow organization imaged with differential interference contrast microscopy, and stress response gene expression in furrow collagen gene mutants at all postembryonic stages. We find that most body shape and furrow disorganization phenotypes start at the L3 stage and increase in severity with each molt afterwards. Stress response genes were induced the strongest in adults, correlating with the greatest Dumpy and furrow phenotypes. Although weaker than in adults, osmolyte transporter gene hmit-1.1 and antimicrobial gene nlp-29 were also induced in some early larvae that had weak or undetectable cuticle phenotypes. Our data are consistent with progressive cuticle phenotypes in which each new cuticle is at least partially directed by organization of the former cuticle. Gene expression and cuticle data support the role of furrow disruption as a signal in L4 larvae and adults, but also suggest a role for other cuticle organization or epidermal cell effects in early larvae.
Keywords: Caenorhabditis elegans; development; extracellular matrix; stress response.
© The Author(s) 2022. Published by Oxford University Press on behalf of Genetics Society of America.
Figures


References
-
- Choe KP. Physiological and molecular mechanisms of salt and water homeostasis in the nematode Caenorhabditis elegans. Am J Physiol Regul Integr Comp Physiol. 2013;305(3):R175–R186. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
