Four Novel Leaderless Bacteriocins, Bacin A1, A2, A3, and A4 Exhibit Potent Antimicrobial and Antibiofilm Activities against Methicillin-Resistant Staphylococcus aureus
- PMID: 36000904
- PMCID: PMC9602277
- DOI: 10.1128/spectrum.00945-22
Four Novel Leaderless Bacteriocins, Bacin A1, A2, A3, and A4 Exhibit Potent Antimicrobial and Antibiofilm Activities against Methicillin-Resistant Staphylococcus aureus
Abstract
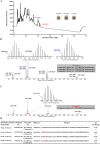
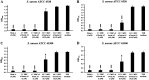
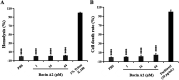
Methicillin-resistant Staphylococcus aureus (MRSA) is a major bacterial pathogen that causes hospital- and community-acquired infections. Owing to its multidrug resistance, it is imperative to develop new antimicrobial agents to treat MRSA infections. In this study, using genome mining analysis and a culture-based screening method to detect bacteriocin activity, we screened a strain, Bacillus sp. TL12, which harbored a putative leaderless bacteriocin gene cluster (bac gene cluster) and exhibited potent anti-MRSA activity. The antimicrobial agents, products of the bac gene cluster, were purified and identified as four novel leaderless bacteriocins: bacin A1, A2, A3, and A4. Bacin A2 was evaluated as a representative antimicrobial agent and showed remarkable antimicrobial activity against S. aureus, MRSA, and the foodborne pathogens Listeria monocytogenes and Bacillus cereus. Mechanistic experiments revealed that bacin A2 damaged cell membranes and exhibited bactericidal activity against MRSA. Bacin A2 effectively inhibited the formation of S. aureus and MRSA biofilms (>0.5× MIC) and killed the cells in their established biofilms (>4× MIC). The hemolytic and NIH/3T3 cytotoxicity assay results for bacin A2 confirmed its biosafety. Thus, bacins have potential as alternative antimicrobial agents for treating MRSA infections. IMPORTANCE Methicillin-resistant Staphylococcus aureus (MRSA) is a major human pathogen that is difficult to treat because of its resistance to several widely used antibiotics. The present study aimed to identify novel anti-MRSA bacteriocins in a prominent producer of bacteriocins, Bacillus cereus group. Four novel leaderless bacteriocins, bacin A1, A2, A3, and A4, which show potent bactericidal effect against S. aureus and MRSA, were identified in Bacillus sp. TL12. Moreover, bacins inhibited biofilm formation and killed cells in the established biofilms of S. aureus and MRSA. These findings suggest that bacins are promising alternatives to treat MRSA infections.
Keywords: bacin; leaderless bacteriocin; methicillin-resistant Staphylococcus aureus (MRSA).
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Thuricins: Novel Leaderless Bacteriocins with Potent Antimicrobial Activity Against Gram-Positive Foodborne Pathogens.J Agric Food Chem. 2022 Aug 17;70(32):9990-9999. doi: 10.1021/acs.jafc.2c02890. Epub 2022 Aug 4. J Agric Food Chem. 2022. PMID: 35924350
-
Ursoricin, a bacteriocin of Streptococcus ursoris, has potent activity against methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci.Appl Environ Microbiol. 2024 Jun 18;90(6):e0016224. doi: 10.1128/aem.00162-24. Epub 2024 May 22. Appl Environ Microbiol. 2024. PMID: 38775468 Free PMC article.
-
Inhibitory effect of Bacillus velezensis 1273 strain cell-free supernatant against developing and preformed biofilms of Staphylococcus aureus and MRSA.Microb Pathog. 2024 Dec;197:107065. doi: 10.1016/j.micpath.2024.107065. Epub 2024 Oct 22. Microb Pathog. 2024. PMID: 39447663
-
Lactic acid bacteria and their bacteriocins: new potential weapons in the fight against methicillin-resistant Staphylococcus aureus.Future Microbiol. 2022 Jun;17:683-699. doi: 10.2217/fmb-2021-0256. Epub 2022 Apr 13. Future Microbiol. 2022. PMID: 35414206 Review.
-
Correlation Between Biofilm Formation and Antibiotic Resistance in MRSA and MSSA Isolated from Clinical Samples in Iran: A Systematic Review and Meta-Analysis.Microb Drug Resist. 2020 Sep;26(9):1071-1080. doi: 10.1089/mdr.2020.0001. Epub 2020 Mar 10. Microb Drug Resist. 2020. PMID: 32159447
Cited by
-
Investigation of the Antibacterial Effectiveness of Hybrid Recombinant Bacteriocins Circular Enterocin and Pyocin L1 Derived from Pseudomonas aeruginosa and Enterococcus faecalis.Biochem Genet. 2025 Jun 13. doi: 10.1007/s10528-025-11149-5. Online ahead of print. Biochem Genet. 2025. PMID: 40512297
-
A Review on Enterocin DD14, the Leaderless Two-Peptide Bacteriocin with Multiple Biological Functions and Unusual Transport Pathway.Antibiotics (Basel). 2023 Jul 14;12(7):1188. doi: 10.3390/antibiotics12071188. Antibiotics (Basel). 2023. PMID: 37508284 Free PMC article. Review.
-
Probiotics and Their Bioproducts: A Promising Approach for Targeting Methicillin-Resistant Staphylococcus aureus and Vancomycin-Resistant Enterococcus.Microorganisms. 2023 Sep 25;11(10):2393. doi: 10.3390/microorganisms11102393. Microorganisms. 2023. PMID: 37894051 Free PMC article. Review.
-
Postbiotics as potential new therapeutic agents for sepsis.Burns Trauma. 2023 Jun 15;11:tkad022. doi: 10.1093/burnst/tkad022. eCollection 2023. Burns Trauma. 2023. PMID: 37334140 Free PMC article. Review.
-
Staphylococcal Drug Resistance: Mechanisms, Therapies, and Nanoparticle Interventions.Infect Drug Resist. 2025 Feb 19;18:1007-1033. doi: 10.2147/IDR.S510024. eCollection 2025. Infect Drug Resist. 2025. PMID: 39990781 Free PMC article. Review.
References
-
- Cassini A, Högberg LD, Plachouras D, Quattrocchi A, Hoxha A, Simonsen GS, Colomb-Cotinat M, Kretzschmar ME, Devleesschauwer B, Cecchini M, Ouakrim DA, Oliveira TC, Struelens MJ, Suetens C, Monnet DL, Strauss R, Mertens K, Struyf T, Catry B, Latour K, Ivanov IN, Dobreva EG, Tambic Andraševic A, Soprek S, Budimir A, Paphitou N, Žemlicková H, Schytte Olsen S, Wolff Sönksen U, Märtin P, Ivanova M, Lyytikäinen O, Jalava J, Coignard B, Eckmanns T, Abu Sin M, Haller S, Daikos GL, Gikas A, Tsiodras S, Kontopidou F, Tóth Á, Hajdu Á, Guólaugsson Ó, Kristinsson KG, Murchan S, Burns K, Pezzotti P, Gagliotti C, Dumpis U, Burden of AMR Collaborative Group, et al. 2019. Attributable deaths and disability adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: a population-level modelling analysis. Lancet Infect Dis 19:56–66. doi: 10.1016/S1473-3099(18)30605-4. - DOI - PMC - PubMed
-
- Baroja I, Guerra S, Coral-Almeida M, Ruíz A, Galarza JM, de Waard JH, Bastidas-Caldes C. 2021. Methicillin-resistant Staphylococcus aureus nasal colonization among health care workers of a tertiary hospital in Ecuador and associated risk factors. IDR 14:3433–3440. doi: 10.2147/IDR.S326148. - DOI - PMC - PubMed
-
- O’Neill J. 2014. Antimicrobial resistance: tackling a crisis for the health and wealth of nations. https://www.naturallivestockfarming.com/wp-content/uploads/2015/09/Antib....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

