A Novel Nanostructured Surface on Titanium Implants Increases Osseointegration in a Sheep Model
- PMID: 36001022
- PMCID: PMC10476811
- DOI: 10.1097/CORR.0000000000002327
A Novel Nanostructured Surface on Titanium Implants Increases Osseointegration in a Sheep Model
Abstract
Background: A nanostructured titanium surface that promotes antimicrobial activity and osseointegration would provide the opportunity to create medical implants that can prevent orthopaedic infection and improve bone integration. Although nanostructured surfaces can exhibit antimicrobial activity, it is not known whether these surfaces are safe and conducive to osseointegration.
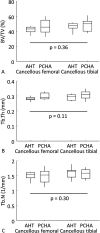
Questions/purposes: Using a sheep animal model, we sought to determine whether the bony integration of medical-grade, titanium, porous-coated implants with a unique nanostructured surface modification (alkaline heat treatment [AHT]) previously shown to kill bacteria was better than that for a clinically accepted control surface of porous-coated titanium covered with hydroxyapatite (PCHA) after 12 weeks in vivo. The null hypothesis was that there would be no difference between implants with respect to the primary outcomes: interfacial shear strength and percent intersection surface (the percentage of implant surface with bone contact, as defined by a micro-CT protocol), and the secondary outcomes: stiffness, peak load, energy to failure, and micro-CT (bone volume/total volume [BV/TV], trabecular thickness [Tb.Th], and trabecular number [Tb.N]) and histomorphometric (bone-implant contact [BIC]) parameters.
Methods: Implants of each material (alkaline heat-treated and hydroxyapatite-coated titanium) were surgically inserted into femoral and tibial metaphyseal cancellous bone (16 per implant type; interference fit) and in tibial cortices at three diaphyseal locations (24 per implant type; line-to-line fit) in eight skeletally mature sheep. At 12 weeks postoperatively, bones were excised to assess osseointegration of AHT and PCHA implants via biomechanical push-through tests, micro-CT, and histomorphometry. Bone composition and remodeling patterns in adult sheep are similar to that of humans, and this model enables comparison of implants with ex vivo outcomes that are not permissible with humans. Comparisons of primary and secondary outcomes were undertaken with linear mixed-effects models that were developed for the cortical and cancellous groups separately and that included a random effect of animals, covariates to adjust for preoperative bodyweight, and implant location (left/right limb, femoral/tibial cancellous, cortical diaphyseal region, and medial/lateral cortex) as appropriate. Significance was set at an alpha of 0.05.
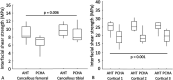
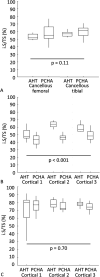
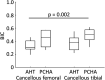
Results: The estimated marginal mean interfacial shear strength for cancellous bone, adjusted for covariates, was 1.6 MPa greater for AHT implants (9.3 MPa) than for PCHA implants (7.7 MPa) (95% CI 0.5 to 2.8; p = 0.006). Similarly, the estimated marginal mean interfacial shear strength for cortical bone, adjusted for covariates, was 6.6 MPa greater for AHT implants (25.5 MPa) than for PCHA implants (18.9 MPa) (95% CI 5.0 to 8.1; p < 0.001). No difference in the implant-bone percent intersection surface was detected for cancellous sites (cancellous AHT 55.1% and PCHA 58.7%; adjusted difference of estimated marginal mean -3.6% [95% CI -8.1% to 0.9%]; p = 0.11). In cortical bone, the estimated marginal mean percent intersection surface at the medial site, adjusted for covariates, was 11.8% higher for AHT implants (58.1%) than for PCHA (46.2% [95% CI 7.1% to 16.6%]; p < 0.001) and was not different at the lateral site (AHT 75.8% and PCHA 74.9%; adjusted difference of estimated marginal mean 0.9% [95% CI -3.8% to 5.7%]; p = 0.70).
Conclusion: These data suggest there is stronger integration of bone on the AHT surface than on the PCHA surface at 12 weeks postimplantation in this sheep model.
Clinical relevance: Given that the AHT implants formed a more robust interface with cortical and cancellous bone than the PCHA implants, a clinical noninferiority study using hip stems with identical geometries can now be performed to compare the same surfaces used in this study. The results of this preclinical study provide an ethical baseline to proceed with such a clinical study given the potential of the alkaline heat-treated surface to reduce periprosthetic joint infection and enhance implant osseointegration.
Copyright © 2022 by the Association of Bone and Joint Surgeons.
Conflict of interest statement
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Figures







Comment in
-
CORR Insights®: A Novel Nanostructured Surface on Titanium Implants Increases Osseointegration in a Sheep Model.Clin Orthop Relat Res. 2022 Nov 1;480(11):2251-2253. doi: 10.1097/CORR.0000000000002365. Epub 2022 Aug 24. Clin Orthop Relat Res. 2022. PMID: 36001013 Free PMC article. No abstract available.
References
-
- Akin FA, Zreiqat H, Jordan S, Wijesundara MBJ, Hanley L. Preparation and analysis of macroporous Ti02 films on Ti surfaces for bone-tissue implants. J Biomed Mater Res. 2001;57:588-596. - PubMed
-
- Arens D, Sigrist I, Alini M, et al. Seasonal changes in bone metabolism in sheep. Vet J. 2007;174:585-591. - PubMed
-
- ASTM International. Standard specification for titanium and titanium-6 aluminum-4 vanadium alloy powders for coatings of surgical implants. ASTM standard F1580-12. Annual Book of ASTM Standards. ASTM International; 2012. DOI: 10.1155/2011/836587. - DOI
-
- ASTM International. Standard specification for wrought titanium-6 aluminum-4 vanadium ELI (extra low interstitial) alloy for surgical implant applications (UNS R56401). ASTM standard F136-13. Annual Book of ASTM Standards. ASTM International; 2013. DOI: 10.1520/F0136-13. - DOI
-
- ASTM International. Standard specification for composition of hydroxylapatite for surgical implants. ASTM standard F1185-03. Annual Book of ASTM Standards . ASTM International; 2014. DOI: 10.1520/F1185-03R14. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

