Cellular basis of differential endochondral growth in Lake Malawi cichlids
- PMID: 36001035
- PMCID: PMC9722610
- DOI: 10.1002/dvdy.529
Cellular basis of differential endochondral growth in Lake Malawi cichlids
Abstract
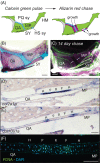
Background: The shape and size of skeletal elements is determined by embryonic patterning mechanisms as well as localized growth and remodeling during post-embryonic development. Differential growth between endochondral growth plates underlies many aspects of morphological diversity in tetrapods but has not been investigated in ray-finned fishes. We examined endochondral growth rates in the craniofacial skeletons of two cichlid species from Lake Malawi that acquire species-specific morphological differences during postembryonic development and quantified cellular mechanisms underlying differential growth both within and between species.
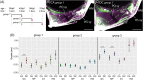
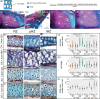
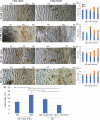
Results: Cichlid endochondral growth rates vary greatly (50%-60%) between different growth zones within a species, between different stages for the same growth zone, and between homologous growth zones in different species. Differences in cell proliferation and/or cell enlargement underlie much of this differential growth, albeit in different proportions. Strikingly, differences in extracellular matrix production do not correlate with growth rate differences.
Conclusions: Differential endochondral growth drives many aspects of craniofacial morphological diversity in cichlids. Cellular proliferation and enlargement, but not extracellular matrix deposition, underlie this differential growth and this appears conserved in Osteichthyes. Cell enlargement is observed in some but not all cichlid growth zones and the degree to which it occurs resembles slower growing mammalian growth plates.
Keywords: cartilage; chondrocyte; craniofacial; hypertrophy; skeleton; teleosts.
© 2022 The Authors. Developmental Dynamics published by Wiley Periodicals LLC on behalf of American Association for Anatomy.
Figures

References
-
- Farnum C. Postnatal Growth of Fins and Limbs through Endochondral Ossification. Chicago: University of Chicago Press; 2007:118‐151.

