Roles of RodZ and class A PBP1b in the assembly and regulation of the peripheral peptidoglycan elongasome in ovoid-shaped cells of Streptococcus pneumoniae D39
- PMID: 36001060
- PMCID: PMC9804626
- DOI: 10.1111/mmi.14969
Roles of RodZ and class A PBP1b in the assembly and regulation of the peripheral peptidoglycan elongasome in ovoid-shaped cells of Streptococcus pneumoniae D39
Abstract
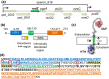
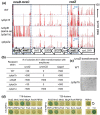
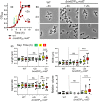
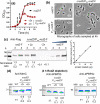
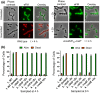
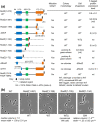
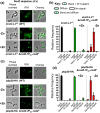
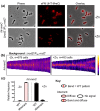
RodZ of rod-shaped bacteria functions to link MreB filaments to the Rod peptidoglycan (PG) synthase complex that moves circumferentially perpendicular to the long cell axis, creating hoop-like sidewall PG. Ovoid-shaped bacteria, such as Streptococcus pneumoniae (pneumococcus; Spn) that lack MreB, use a different modality for peripheral PG elongation that emanates from the midcell of dividing cells. Yet, S. pneumoniae encodes a RodZ homolog similar to RodZ in rod-shaped bacteria. We show here that the helix-turn-helix and transmembrane domains of RodZ(Spn) are essential for growth at 37°C. ΔrodZ mutations are suppressed by Δpbp1a, mpgA(Y488D), and ΔkhpA mutations that suppress ΔmreC, but not ΔcozE. Consistent with a role in PG elongation, RodZ(Spn) co-localizes with MreC and aPBP1a throughout the cell cycle and forms complexes and interacts with PG elongasome proteins and regulators. Depletion of RodZ(Spn) results in aberrantly shaped, non-growing cells and mislocalization of elongasome proteins MreC, PBP2b, and RodA. Moreover, Tn-seq reveals that RodZ(Spn), but not MreCD(Spn), displays a specific synthetic-viable genetic relationship with aPBP1b, whose function is unknown. We conclude that RodZ(Spn) acts as a scaffolding protein required for elongasome assembly and function and that aPBP1b, like aPBP1a, plays a role in elongasome regulation and possibly peripheral PG synthesis.
Keywords: class A PBP function and regulation; elongasome assembly; peptidoglycan synthesis; synthetic-viable genetic relationships.
© 2022 The Authors. Molecular Microbiology published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no conflicts of interests.
Figures




References
-
- Aaron, M. , Charbon, G. , Lam, H. , Schwarz, H. , Vollmer, W. & Jacobs‐Wagner, C. (2007) The tubulin homologue FtsZ contributes to cell elongation by guiding cell wall precursor synthesis in Caulobacter crescentus . Molecular Microbiology, 64, 938–952. - PubMed
-
- Aravind, L. , Anantharaman, V. , Balaji, S. , Babu, M.M. & Iyer, L.M. (2005) The many faces of the helix‐turn‐helix domain: transcription regulation and beyond. FEMS Microbiology Reviews, 29, 231–262. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

