Developmental stage-specific spontaneous activity contributes to callosal axon projections
- PMID: 36001081
- PMCID: PMC9402231
- DOI: 10.7554/eLife.72435
Developmental stage-specific spontaneous activity contributes to callosal axon projections
Abstract
The developing neocortex exhibits spontaneous network activity with various synchrony levels, which has been implicated in the formation of cortical circuits. We previously reported that the development of callosal axon projections, one of the major long-range axonal projections in the brain, is activity dependent. However, what sort of activity and when activity is indispensable are not known. Here, using a genetic method to manipulate network activity in a stage-specific manner, we demonstrated that network activity contributes to callosal axon projections in the mouse visual cortex during a 'critical period': restoring neuronal activity during that period resumed the projections, whereas restoration after the period failed. Furthermore, in vivo Ca2+ imaging revealed that the projections could be established even without fully restoring highly synchronous activity. Overall, our findings suggest that spontaneous network activity is selectively required during a critical developmental time window for the formation of long-range axonal projections in the cortex.
Keywords: activity dependent; cerebral cortex; development; mouse; neuroscience.
© 2022, Tezuka, Hagihara et al.
Conflict of interest statement
YT, KH, KO, TH, YT No competing interests declared
Figures

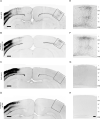
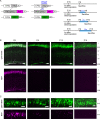
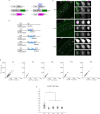




References
-
- Alexander GM, Rogan SC, Abbas AI, Armbruster BN, Pei Y, Allen JA, Nonneman RJ, Hartmann J, Moy SS, Nicolelis MA, McNamara JO, Roth BL. Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. Neuron. 2009;63:27–39. doi: 10.1016/j.neuron.2009.06.014. - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Miscellaneous

