Prescribing Patterns of Codeine and Alternative Medicines in Children in Europe
- PMID: 36001288
- PMCID: PMC9492592
- DOI: 10.1007/s40264-022-01214-y
Prescribing Patterns of Codeine and Alternative Medicines in Children in Europe
Abstract
Introduction: Concerns over serious respiratory depression in children led to two European Union (EU) referral procedures (in 2013 and 2015) to review the benefit-risk balance of codeine in this population when used for pain relief, cough or cold. Consequently, codeine should no longer be used in children aged < 12 years and restrictions were introduced for treatment in children ≥ 12 years.
Objective: This multinational collaborative study aimed to assess the effectiveness of these risk minimisation measures by evaluating changes in prescribing of codeine and alternative treatments.
Method: Children under 12 and 12-18 years old were followed between 2010 and 2017 to analyse quarterly trends in prescribing of codeine and alternative treatments in electronic health records from France, Germany, Norway, Spain and the United Kingdom using interrupted time series analysis.
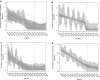
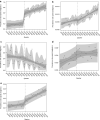
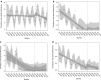
Results: Overall prescribing of codeine in children decreased in all five countries, reaching near zero prevalence in children under 12 years of age. This was accompanied by an increase in use of other opioid analgesics in France (from 0.15 to 0.56 prevalence per 100 person-years immediately after the first referral), Norway (from 0.0006 to 0.0013 at the end of the study), the United Kingdom (from 0.018 to 0.05 at the end of the study), and an increase in non-opioid analgesics in Norway (from 0.045 to 0.075 at the end of the study) after the referral on pain relief indication. The referral on cough/cold indication led to a decrease in use of opioid and non-opioid antitussives in children aged < 12 years in France (from 10 to 7 and 20 to 16, respectively) and had no impact in other countries. Overall prescribing trends for codeine and alternatives were similar across both age groups within each country.
Conclusion: The decrease in use of codeine shows that healthcare professionals followed the adopted measures and switched prescribing practices for pain management in children aged < 18 years towards opioid or non-opioid analgesics depending on national clinical and reimbursement settings. Whist the magnitude of the first referral on pain differed between countries, the second referral on cough/cold had only a minimal impact on the use of codeine and antitussives.
© 2022. The Author(s).
Conflict of interest statement
The authors declared no competing interests for this work. The views expressed in this article are the personal views of the author(s) and may not be understood or quoted as being made on behalf of or reflecting the position of the regulatory agency/agencies or organisations with which the author(s) is/are employed/affiliated. BIFAP is a public program for independent research financed by the Spanish Agency for Medicines and Medical Devices (AEMPS). The results, discussion, and conclusions of this work are only of the authors and do not represent in any way the position of the AEMPS on this subject. The excellent collaboration of the primary care physicians (general practitioners/paediatricians) as well as the support from the regional health administrations providing BIFAP data is acknowledged.
Figures



Similar articles
-
A European multicentre drug utilisation study of the impact of regulatory measures on prescribing of codeine for pain in children.Pharmacoepidemiol Drug Saf. 2019 Aug;28(8):1086-1096. doi: 10.1002/pds.4836. Epub 2019 Jun 20. Pharmacoepidemiol Drug Saf. 2019. PMID: 31219227 Free PMC article.
-
Codeine for acute cough in children.Can Fam Physician. 2010 Dec;56(12):1293-4. Can Fam Physician. 2010. PMID: 21156892 Free PMC article.
-
Codeine: A Relook at the Old Antitussive.J Assoc Physicians India. 2015 Apr;63(4):80, 82-5. J Assoc Physicians India. 2015. PMID: 26591180
-
Codeine and its alternates for pain and cough relief. 2. Alternates for pain relief.Bull World Health Organ. 1969;40(1):1-53. Bull World Health Organ. 1969. PMID: 4894737 Free PMC article. Review.
-
[New insights into the role of pholcodine in the treatment of cough in 2013?].Therapie. 2013 Mar-Apr;68(2):85-91. doi: 10.2515/therapie/2013019. Epub 2013 Jun 18. Therapie. 2013. PMID: 23773349 Review. French.
References
-
- List of European Union reference dates and frequency of submission of periodic safety update reports (PSURs). https://www.ema.europa.eu/documents/other/list-european-union-reference-.... Accessed 16 June 2022.
-
- Dean L, Kane M. Codeine therapy and CYP2D6 genotype. 2012 Sep 20 [updated 2021 Mar 30]. In: Pratt VM, Scott SA, Pirmohamed M, Esquivel B, Kane MS, Kattman BL, Malheiro AJ, editors. Medical genetics summaries [Internet]. Bethesda: National Center for Biotechnology Information (US); 2012 (PMID: 28520350). - PubMed
-
- Codeine: very rare risk of side-effects in breastfed babies. Medicines and Healthcare products Regulatory Agency (MHRA) website. https://www.gov.uk/drug-safety-update/codeine-very-rare-risk-of-side-eff.... Accessed 16 June 2022.
-
- Safety review update of codeine use in children; new Boxed Warning and Contraindication on use after tonsillectomy and/or adenoidectomy. U.S. Food and Drug Administration. https://www.fda.gov/media/85072/download#:~:text=In%20August%202012%2C%2.... Accessed 16 June 2022.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

