Midazolam, methamphetamine, morphine and nicotine intake in high-drinking-in-the-dark mice
- PMID: 36001437
- PMCID: PMC9677807
- DOI: 10.1111/adb.13212
Midazolam, methamphetamine, morphine and nicotine intake in high-drinking-in-the-dark mice
Abstract
The high-drinking-in-the-dark (HDID) lines of mice were selectively bred for achieving high blood alcohol levels in the drinking-in-the-dark (DID) task and have served as a unique genetic risk model for binge-like alcohol intake. However, little is known about their willingness to consume other addictive drugs. Here, we examined (a) whether the HDID-1 and HDID-2 lines of mice would voluntarily consume midazolam, methamphetamine, morphine and nicotine in a DID test and (b) whether the HDID lines differ from their founders, heterogeneous stock/Northport (HS/NPT), in consumption levels of these drugs at the concentrations tested. Separate groups of HDID-1, HDID-2 and HS/NPT mice were given 4 days of access to each drug, using the single-bottle, limited-access DID paradigm. Male and female mice of both HDID lines consumed all four offered drugs. We observed no genotype differences in 40 μg/ml methamphetamine intake, but significant differences in nicotine, midazolam and morphine intake. Both HDID lines drank significantly more (150 μg/ml) midazolam than their founders, providing strong support for a shared genetic contribution to binge ethanol and midazolam intake. HDID-2 mice, but not HDID-1 mice, consumed more morphine (700 μg/ml) and more nicotine across a range of concentrations than HS/NPT mice. These results demonstrate that the HDID mice can be utilized for tests of voluntary drug consumption other than ethanol and highlight potentially important differences between HDID lines in risk for elevated drug intake.
Keywords: DID; HDID; methamphetamine; midazolam; morphine; nicotine.
© 2022 Society for the Study of Addiction.
Figures
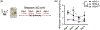
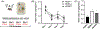
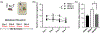
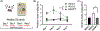
Similar articles
-
The impact of Drinking in the Dark (DID) procedural manipulations on ethanol intake in High Drinking in the Dark (HDID) mice.Alcohol. 2021 Jun;93:45-56. doi: 10.1016/j.alcohol.2021.02.001. Epub 2021 Feb 6. Alcohol. 2021. PMID: 33556460 Free PMC article.
-
Alcohol preference drinking in a mouse line selectively bred for high drinking in the dark.Alcohol. 2011 Aug;45(5):427-40. doi: 10.1016/j.alcohol.2010.12.001. Epub 2010 Dec 30. Alcohol. 2011. PMID: 21194877 Free PMC article.
-
Ethanol drinking microstructure of a high drinking in the dark selected mouse line.Alcohol Clin Exp Res. 2012 Aug;36(8):1330-9. doi: 10.1111/j.1530-0277.2012.01749.x. Epub 2012 Apr 23. Alcohol Clin Exp Res. 2012. PMID: 22524154 Free PMC article.
-
High drinking in the dark mice: a genetic model of drinking to intoxication.Alcohol. 2014 May;48(3):217-23. doi: 10.1016/j.alcohol.2013.10.007. Epub 2013 Nov 15. Alcohol. 2014. PMID: 24360287 Free PMC article. Review.
-
Scheduled access alcohol drinking by alcohol-preferring (P) and high-alcohol-drinking (HAD) rats: modeling adolescent and adult binge-like drinking.Alcohol. 2014 May;48(3):225-34. doi: 10.1016/j.alcohol.2013.10.004. Epub 2013 Oct 31. Alcohol. 2014. PMID: 24290311 Free PMC article. Review.
References
-
- Saha TD, Grant BF, Chou SP, Kerridge BT, Pickering RP, Ruan WJ. Concurrent use of alcohol with other drugs and DSM-5 alcohol use disorder comorbid with other drug use disorders: sociodemographic characteristics, severity, and psychopathology. Drug Alcohol Depend 2018;187:261–269. doi:10.1016/j.drugalcdep.2018.03.006 - DOI - PubMed
-
- Stinson FS, Grant BF, Dawson DA, Ruan WJ, Huang B, Saha T. Comorbidity between DSM-IV alcohol and specific drug use disorders in the United States: results from the National Epidemiologic Survey on alcohol and related conditions. Drug Alcohol Depend 2005;80(1): 105–116. doi:10.1016/j.drugalcdep.2005.03.009 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

