Effects of varenicline and bupropion on laboratory smoking outcomes: Meta-analysis of randomized, placebo-controlled human laboratory studies
- PMID: 36001439
- PMCID: PMC9413474
- DOI: 10.1111/adb.13218
Effects of varenicline and bupropion on laboratory smoking outcomes: Meta-analysis of randomized, placebo-controlled human laboratory studies
Abstract
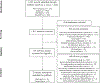
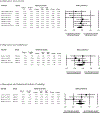
Human laboratory studies are widely used to evaluate behavioural mechanisms of pharmacotherapy effects. Results from human laboratory studies examining smoking cessation pharmacotherapies have not been examined in aggregate. The current meta-analysis aimed to synthesize data from randomized, placebo-controlled human laboratory studies on the effects of non-nicotine pharmacotherapies on outcomes relevant for smoking cessation. Literature searches identified 15 human laboratory studies of varenicline (n = 697) and 9 studies of bupropion (n = 313) with sufficient data for inclusion. Studies involved acute or subacute pharmacotherapy treatment with administration durations ranging from a single dose to 8 weeks. Primary outcomes examined were craving, withdrawal and behavioural indices of smoking. Varenicline significantly reduced craving (Hedge's g = -0.36[-0.54,-0.17], p < 0.001), withdrawal (g = -0.25[-0.41,-0.09], p = 0.003) and behavioural indices of smoking (g = -0.36[-0.63,-0.08], p = 0.01) relative to placebo. In contrast, results were inconclusive regarding bupropion's effects on craving (g = -0.13[-0.32,0.05], p = 0.15), withdrawal (g = -0.15[-0.44,0.14], p = 0.31) and behavioural indices of smoking (g = -0.05[-0.35,0.24], p = 0.73) relative to placebo. Findings provide meta-analytic support that short-term varenicline treatment decreases craving, withdrawal symptoms and smoking behaviour under controlled laboratory conditions. However, findings also suggest the ability of human laboratory paradigms to detect pharmacotherapy effects may differ by treatment type. Pharmacotherapy discovery and evaluation efforts utilizing human laboratory methods should aim to align study designs and laboratory procedures with presumed therapeutic mechanisms when possible.
Keywords: bupropion; tobacco use disorder; varenicline.
© 2022 Society for the Study of Addiction.
Figures

Similar articles
-
Effect of varenicline and bupropion SR on craving, nicotine withdrawal symptoms, and rewarding effects of smoking during a quit attempt.Psychopharmacology (Berl). 2008 Apr;197(3):371-7. doi: 10.1007/s00213-007-1041-3. Epub 2007 Dec 15. Psychopharmacology (Berl). 2008. PMID: 18084743
-
Varenicline: a first-line treatment option for smoking cessation.Clin Ther. 2009 Mar;31(3):463-91. doi: 10.1016/j.clinthera.2009.03.021. Clin Ther. 2009. PMID: 19393839 Review.
-
Pharmacotherapies for smoking cessation: a meta-analysis of randomized controlled trials.CMAJ. 2008 Jul 15;179(2):135-44. doi: 10.1503/cmaj.070256. CMAJ. 2008. PMID: 18625984 Free PMC article. Review.
-
Bupropion for the treatment of nicotine withdrawal and craving.Expert Rev Neurother. 2006 Jul;6(7):965-81. doi: 10.1586/14737175.6.7.965. Expert Rev Neurother. 2006. PMID: 16831112 Review.
-
Effects of varenicline and bupropion sustained-release use plus intensive smoking cessation counseling on prolonged abstinence from smoking and on depression, negative affect, and other symptoms of nicotine withdrawal.JAMA Psychiatry. 2013 May;70(5):522-33. doi: 10.1001/jamapsychiatry.2013.678. JAMA Psychiatry. 2013. PMID: 23536105 Free PMC article. Clinical Trial.
Cited by
-
Bupropion Mediated Effects on Depression, Attention Deficit Hyperactivity Disorder, and Smoking Cessation.Health Psychol Res. 2023 Jul 1;11:81043. doi: 10.52965/001c.81043. eCollection 2023. Health Psychol Res. 2023. PMID: 37405312 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

