Inhibition of PSD95-nNOS protein-protein interactions decreases morphine reward and relapse vulnerability in rats
- PMID: 36001441
- PMCID: PMC9539577
- DOI: 10.1111/adb.13220
Inhibition of PSD95-nNOS protein-protein interactions decreases morphine reward and relapse vulnerability in rats
Abstract
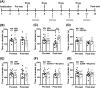
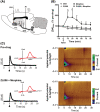
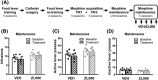
Glutamate signalling through the N-methyl-d-aspartate receptor (NMDAR) activates the enzyme neuronal nitric oxide synthase (nNOS) to produce the signalling molecule nitric oxide (NO). We hypothesized that disruption of the protein-protein interaction between nNOS and the scaffolding protein postsynaptic density 95 kDa (PSD95) would block NMDAR-dependent NO signalling and represent a viable therapeutic route to decrease opioid reward and relapse-like behaviour without the unwanted side effects of NMDAR antagonists. We used a conditioned place preference (CPP) paradigm to evaluate the impact of two small-molecule PSD95-nNOS inhibitors, IC87201 and ZL006, on the rewarding effects of morphine. Both IC87201 and ZL006 blocked morphine-induced CPP at doses that lacked intrinsic rewarding or aversive properties. Furthermore, in vivo fast-scan cyclic voltammetry (FSCV) was used to ascertain the impact of ZL006 on morphine-induced increases in dopamine (DA) efflux in the nucleus accumbens shell (NAc shell) evoked by electrical stimulation of the medial forebrain bundle (MFB). ZL006 attenuated morphine-induced increases in DA efflux at a dose that did not have intrinsic effects on DA transmission. We also employed multiple intravenous drug self-administration approaches to examine the impact of ZL006 on the reinforcing effects of morphine. Interestingly, ZL006 did not alter acquisition or maintenance of morphine self-administration, but reduced lever pressing in a morphine relapse test after forced abstinence. Our results provide behavioural and neurochemical support for the hypothesis that inhibition of PSD95-nNOS protein-protein interactions decreases morphine reward and relapse-like behaviour, highlighting a previously unreported application for these novel therapeutics in the treatment of opioid addiction.
Keywords: PSD95; nNOS; nitric oxide; protein-protein interaction; relapse; reward.
© 2022 The Authors. Addiction Biology published by John Wiley & Sons Ltd on behalf of Society for the Study of Addiction.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures


Similar articles
-
Small molecule inhibitors of PSD95-nNOS protein-protein interactions suppress formalin-evoked Fos protein expression and nociceptive behavior in rats.Neuroscience. 2017 May 4;349:303-317. doi: 10.1016/j.neuroscience.2017.02.055. Epub 2017 Mar 8. Neuroscience. 2017. PMID: 28285942 Free PMC article.
-
Small molecule inhibitors of PSD95-nNOS protein-protein interactions as novel analgesics.Neuropharmacology. 2015 Oct;97:464-75. doi: 10.1016/j.neuropharm.2015.05.038. Epub 2015 Jun 10. Neuropharmacology. 2015. PMID: 26071110 Free PMC article.
-
Source memory in rats is impaired by an NMDA receptor antagonist but not by PSD95-nNOS protein-protein interaction inhibitors.Behav Brain Res. 2016 May 15;305:23-9. doi: 10.1016/j.bbr.2016.02.021. Epub 2016 Feb 22. Behav Brain Res. 2016. PMID: 26909849 Free PMC article.
-
PSD95 and nNOS interaction as a novel molecular target to modulate conditioned fear: relevance to PTSD.Transl Psychiatry. 2018 Aug 14;8(1):155. doi: 10.1038/s41398-018-0208-5. Transl Psychiatry. 2018. PMID: 30108200 Free PMC article.
-
Localization of brain reinforcement mechanisms: intracranial self-administration and intracranial place-conditioning studies.Behav Brain Res. 1999 Jun;101(2):129-52. doi: 10.1016/s0166-4328(99)00022-4. Behav Brain Res. 1999. PMID: 10372570 Review.
Cited by
-
Negative allosteric modulation of CB1 cannabinoid receptor signaling suppresses opioid-mediated tolerance and withdrawal without blocking opioid antinociception.Neuropharmacology. 2024 Oct 1;257:110052. doi: 10.1016/j.neuropharm.2024.110052. Epub 2024 Jun 25. Neuropharmacology. 2024. PMID: 38936657 Free PMC article.
-
Metabolite classification through novel metabolomics framework reveals mechanism underlying the therapeutic effects of PSD95-nNOS blockade for post-stroke depression.Metabolomics. 2025 Jul 11;21(4):100. doi: 10.1007/s11306-025-02306-3. Metabolomics. 2025. PMID: 40643722
-
Elevating levels of the endocannabinoid 2-arachidonoylglycerol blunts opioid reward but not analgesia.Sci Adv. 2024 Nov 29;10(48):eadq4779. doi: 10.1126/sciadv.adq4779. Epub 2024 Nov 29. Sci Adv. 2024. PMID: 39612328 Free PMC article.
-
Elevating levels of the endocannabinoid 2-arachidonoylglycerol blunts opioid reward but not analgesia.bioRxiv [Preprint]. 2024 Apr 2:2024.04.02.585967. doi: 10.1101/2024.04.02.585967. bioRxiv. 2024. Update in: Sci Adv. 2024 Nov 29;10(48):eadq4779. doi: 10.1126/sciadv.adq4779. PMID: 38766079 Free PMC article. Updated. Preprint.
-
The cannabinoid CB2 agonist LY2828360 suppresses neuropathic pain behavior and attenuates morphine tolerance and conditioned place preference in rats.Neuropharmacology. 2025 Mar 1;265:110257. doi: 10.1016/j.neuropharm.2024.110257. Epub 2024 Dec 5. Neuropharmacology. 2025. PMID: 39644993
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

