Nirmatrelvir Use and Severe Covid-19 Outcomes during the Omicron Surge
- PMID: 36001529
- PMCID: PMC9454652
- DOI: 10.1056/NEJMoa2204919
Nirmatrelvir Use and Severe Covid-19 Outcomes during the Omicron Surge
Abstract
Background: The oral protease inhibitor nirmatrelvir has shown substantial efficacy in high-risk, unvaccinated patients infected with the B.1.617.2 (delta) variant of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Data regarding the effectiveness of nirmatrelvir in preventing severe coronavirus disease 2019 (Covid-19) outcomes from the B.1.1.529 (omicron) variant are limited.
Methods: We obtained data for all members of Clalit Health Services who were 40 years of age or older at the start of the study period and were assessed as being eligible to receive nirmatrelvir therapy during the omicron surge. A Cox proportional-hazards regression model with time-dependent covariates was used to estimate the association of nirmatrelvir treatment with hospitalization and death due to Covid-19, with adjustment for sociodemographic factors, coexisting conditions, and previous SARS-CoV-2 immunity status.
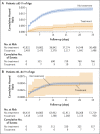
Results: A total of 109,254 patients met the eligibility criteria, of whom 3902 (4%) received nirmatrelvir during the study period. Among patients 65 years of age or older, the rate of hospitalization due to Covid-19 was 14.7 cases per 100,000 person-days among treated patients as compared with 58.9 cases per 100,000 person-days among untreated patients (adjusted hazard ratio, 0.27; 95% confidence interval [CI], 0.15 to 0.49). The adjusted hazard ratio for death due to Covid-19 was 0.21 (95% CI, 0.05 to 0.82). Among patients 40 to 64 years of age, the rate of hospitalization due to Covid-19 was 15.2 cases per 100,000 person-days among treated patients and 15.8 cases per 100,000 person-days among untreated patients (adjusted hazard ratio, 0.74; 95% CI, 0.35 to 1.58). The adjusted hazard ratio for death due to Covid-19 was 1.32 (95% CI, 0.16 to 10.75).
Conclusions: Among patients 65 years of age or older, the rates of hospitalization and death due to Covid-19 were significantly lower among those who received nirmatrelvir than among those who did not. No evidence of benefit was found in younger adults.
Copyright © 2022 Massachusetts Medical Society.
Figures
Comment in
-
Nirmatrelvir Use during the Omicron Surge.N Engl J Med. 2022 Dec 29;387(26):2479. doi: 10.1056/NEJMc2213868. N Engl J Med. 2022. PMID: 36577106 No abstract available.
-
Nirmatrelvir Use during the Omicron Surge.N Engl J Med. 2022 Dec 29;387(26):2479-2480. doi: 10.1056/NEJMc2213868. N Engl J Med. 2022. PMID: 36577107 No abstract available.
-
Nirmatrelvir Use during the Omicron Surge.N Engl J Med. 2022 Dec 29;387(26):2480. doi: 10.1056/NEJMc2213868. N Engl J Med. 2022. PMID: 36577108 No abstract available.
-
Nirmatrelvir Use during the Omicron Surge. Reply.N Engl J Med. 2022 Dec 29;387(26):2480-2481. doi: 10.1056/NEJMc2213868. N Engl J Med. 2022. PMID: 36577109 No abstract available.
-
Nirmatrelvir wirkt auch gegen Omikron.MMW Fortschr Med. 2023 Feb;165(2):26. doi: 10.1007/s15006-023-2290-8. MMW Fortschr Med. 2023. PMID: 36703052 Free PMC article. Review. German. No abstract available.
References
-
- Rubin EJ, Baden LR, Morrissey S. Audio interview: understanding the omicron variant of SARS-CoV-2. N Engl J Med 2022;386(8):e27-e27. - PubMed
-
- Food and Drug Administration. Coronavirus (COVID-19) update: FDA authorizes first oral antiviral for treatment of COVID-19. December 22, 2021. (https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19...).
-
- Food and Drug Administration. Fact sheet for healthcare providers: emergency use authorization for Paxlovid. December 2021. (https://www.fda.gov/media/155050/download).
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
