Clinical manifestations and disease severity of SARS-CoV-2 infection among infants in Canada
- PMID: 36001553
- PMCID: PMC9401116
- DOI: 10.1371/journal.pone.0272648
Clinical manifestations and disease severity of SARS-CoV-2 infection among infants in Canada
Abstract
Background: There are limited data on outcomes of SARS-CoV-2 infection among infants (<1 year of age). In the absence of approved vaccines for infants, understanding characteristics associated with hospitalization and severe disease from COVID-19 in this age group will help inform clinical management and public health interventions. The objective of this study was to describe the clinical manifestations, disease severity, and characteristics associated with hospitalization among infants infected with the initial strains of SARS-CoV-2.
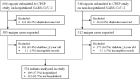
Methods: This is a national, prospective study of infants with SARS-CoV-2 from April 8th 2020 to May 31st 2021 using the infrastructure of the Canadian Paediatric Surveillance Program. Infants <1 year of age with microbiologically confirmed SARS-CoV-2 infection from both inpatients and outpatients seen in clinics and emergency departments were included. Cases were classified as either: 1) Non-hospitalized patient with SARS-CoV-2 infection; 2) COVID-19-related hospitalization; or 3) non-COVID-19-related hospitalization (e.g., incidentally detected SARS-CoV-2). Case severity was defined as asymptomatic, outpatient care, mild (inpatient care), moderate or severe disease. Multivariable logistic regression was performed to identify characteristics associated with hospitalization.
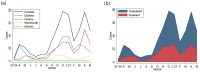
Results: A total of 531 cases were reported, including 332 (62.5%) non-hospitalized and 199 (37.5%) hospitalized infants. Among hospitalized infants, 141 of 199 infants (70.9%) were admitted because of COVID-19-related illness, and 58 (29.1%) were admitted for reasons other than acute COVID-19. Amongst all cases with SARS-CoV-2 infection, the most common presenting symptoms included fever (66.5%), coryza (47.1%), cough (37.3%) and decreased oral intake (25.0%). In our main analysis, infants with a comorbid condition had higher odds of hospitalization compared to infants with no comorbid conditions (aOR = 4.53, 2.06-9.97), and infants <1 month had higher odds of hospitalization then infants aged 1-3 months (aOR = 3.78, 1.97-7.26). In total, 20 infants (3.8%) met criteria for severe disease.
Conclusions: We describe one of the largest cohorts of infants with SARS-CoV-2 infection. Overall, severe COVID-19 in this age group was found to be uncommon. Comorbid conditions and younger age were associated with COVID-19-related hospitalization amongst infants.
Conflict of interest statement
JP reports grants to his institution from MedImmune, Merck, Sanofi Pasteur and AbbVie, and speaker fees from AbbVie and AstraZeneca, all outside of the submitted work. MS is supported via salary awards from the BC Children’s Hospital Foundation, the Canadian Child Health Clinician Scientist Program and the Michael Smith Foundation for Health Research. MS has been an investigator on projects funded by GlaxoSmithKline, Merck, Pfizer, Sanofi-Pasteur, Seqirus, Symvivo and VBI Vaccines. All funds have been paid to his institute, and he has not received any personal payments. PPPR has been co-investigator on an investigator-led project funded by Pfizer that is unrelated to this study. RP is a consultant for Verity Pharmaceuticals. SKM is co-principal investigator on an investigator-led grant from Pfizer, has served on an ad-hoc advisory boards for Pfizer and Sanofi Pasteur, and has received speaker fees from GlaxoSmithKline, all unrelated to this study. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures
Similar articles
-
Characteristics of children admitted to hospital with acute SARS-CoV-2 infection in Canada in 2020.CMAJ. 2021 Sep 27;193(38):E1483-E1493. doi: 10.1503/cmaj.210053. CMAJ. 2021. PMID: 34580141 Free PMC article.
-
Outcomes of SARS-CoV-2-Positive Youths Tested in Emergency Departments: The Global PERN-COVID-19 Study.JAMA Netw Open. 2022 Jan 4;5(1):e2142322. doi: 10.1001/jamanetworkopen.2021.42322. JAMA Netw Open. 2022. PMID: 35015063 Free PMC article.
-
Virtualized clinical studies to assess the natural history and impact of gut microbiome modulation in non-hospitalized patients with mild to moderate COVID-19 a randomized, open-label, prospective study with a parallel group study evaluating the physiologic effects of KB109 on gut microbiota structure and function: a structured summary of a study protocol for a randomized controlled study.Trials. 2021 Apr 2;22(1):245. doi: 10.1186/s13063-021-05157-0. Trials. 2021. PMID: 33810796 Free PMC article.
-
Community-Onset Severe Acute Respiratory Syndrome Coronavirus 2 Infection in Young Infants: A Systematic Review.J Pediatr. 2021 Jan;228:94-100.e3. doi: 10.1016/j.jpeds.2020.09.008. Epub 2020 Sep 8. J Pediatr. 2021. PMID: 32910943 Free PMC article.
-
Clinical features of infants with SARS-CoV-2 infection: a systematic review and meta-analysis.Ann Palliat Med. 2022 Nov;11(11):3394-3408. doi: 10.21037/apm-22-933. Epub 2022 Oct 31. Ann Palliat Med. 2022. PMID: 36366895
Cited by
-
Clinical Course, Laboratory Findings, and Prognosis of SARS-CoV-2 Infection in Infants up to 90 Days of Age: A Single-Center Experience and a Proposal for a Management Pathway.Healthcare (Basel). 2024 Feb 23;12(5):528. doi: 10.3390/healthcare12050528. Healthcare (Basel). 2024. PMID: 38470638 Free PMC article.
-
Clinical Course and Severity of COVID-19 in 940 Infants with and without Comorbidities Hospitalized in 2020 and 2021: The Results of the National Multicenter Database SARSTer-PED.J Clin Med. 2023 Mar 24;12(7):2479. doi: 10.3390/jcm12072479. J Clin Med. 2023. PMID: 37048562 Free PMC article.
-
Serious Bacterial Infection Risk in Febrile Infants Aged ≤90 Days With COVID-19.Cureus. 2025 Jul 14;17(7):e87938. doi: 10.7759/cureus.87938. eCollection 2025 Jul. Cureus. 2025. PMID: 40821183 Free PMC article.
-
COVID-19 among infants: key clinical features and remaining controversies.Clin Exp Pediatr. 2024 Jan;67(1):1-16. doi: 10.3345/cep.2023.00794. Epub 2023 Nov 27. Clin Exp Pediatr. 2024. PMID: 38013408 Free PMC article.
-
Severe and invasive bacterial infections in infants aged less than 90 days with and without SARS-CoV-2 infection.Ital J Pediatr. 2024 Aug 15;50(1):148. doi: 10.1186/s13052-024-01721-x. Ital J Pediatr. 2024. PMID: 39143644 Free PMC article.
References
-
- Tostmann A, Bradley J, Bousema T, et al.. Strong associations and moderate predictive value of early symptoms for SARS-CoV-2 test positivity among healthcare workers, the Netherlands, March 2020. Eurosurveillance. 23 avr 2020;25(16):2000508. doi: 10.2807/1560-7917.ES.2020.25.16.2000508 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

