Tocilizumab, netakimab, and baricitinib in patients with mild-to-moderate COVID-19: An observational study
- PMID: 36001576
- PMCID: PMC9401152
- DOI: 10.1371/journal.pone.0273340
Tocilizumab, netakimab, and baricitinib in patients with mild-to-moderate COVID-19: An observational study
Abstract
Objective: The aim of the study was to assess inflammatory markers and clinical outcomes in adult patients admitted to hospital with mild-to-moderate COVID-19 and treated with a combination of standard-of-care (SOC) and targeted immunosuppressive therapy including anti-IL-17A (netakimab), anti-IL-6R (tocilizumab), or JAK1/JAK2 inhibitor (baricitinib) or with a standard-of-care therapy alone.
Methods: The observational cohort study included 154 adults hospitalized between February and August, 2020 with RT-PCR-confirmed SARS-CoV-2 with National Early Warning Score2 (NEWS2) < 7 and C-reactive protein (CRP) levels ≤ 140 mg/L on the day of the start of the therapy or observation. Patients were divided into the following groups: I) 4 mg baricitinib, 1 or 2 times a day for an average of 5 days (n = 38); II) 120 mg netakimab, one dose (n = 48); III) 400 mg tocilizumab, one dose (n = 34), IV) SOC only: hydroxychloroquine, antiviral, antibacterial, anticoagulant, and dexamethasone (n = 34).
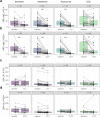
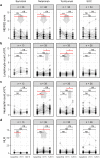
Results: CRP levels significantly decreased after 72 h in the tocilizumab (p = 1 x 10-5) and netakimab (p = 8 x 10-4) groups and remained low after 120 h. The effect was stronger with tocilizumab compared to other groups (p = 0.028). A significant decrease in lactate dehydrogenase (LDH) levels was observed 72 h after netakimab therapy (p = 0.029). NEWS2 scores significantly improved 72 h after tocilizumab (p = 6.8 x 10-5) and netakimab (p = 0.01) therapy, and 120 h after the start of tocilizumab (p = 8.6 x 10-5), netakimab (p = 0.001), or baricitinib (p = 4.6 x 10-4) therapy, but not in the SOC group. Blood neutrophil counts (p = 6.4 x 10-4) and neutrophil-to-lymphocyte ratios (p = 0.006) significantly increased 72 h after netakimab therapy and remained high after 120 h. The percentage of patients discharged 5-7 days after the start of therapy was higher in the tocilizumab (44.1%) and netakimab (41.7%) groups than in the baricitinib (31.6%) and SOC (23.5%) groups. Compared to SOC (3 of the 34; 8.8%), mortality was lower in netakimab (0 of the 48; 0%, RR = 0.1 (95% CI: 0.0054 to 1.91)), tocilizumab (0 of the 34; 0%, RR = 0.14 (95% CI: 0.0077 to 2.67)), and baricitinib (1 of the 38; 2.6%, RR = 0.3 (95% CI: 0.033 to 2.73)) groups.
Conclusion: In hospitalized patients with mild-to-moderate COVID-19, the combination of SOC with anti-IL-17A or anti-IL-6R therapy were superior or comparable to the combination with JAK1/JAK2 inhibitor, and all three were superior to SOC alone. Whereas previous studies did not demonstrate significant benefit of anti-IL-17A therapy for severe COVID-19, our data suggest that such therapy could be a rational choice for mild-to-moderate disease, considering the generally high safety profile of IL-17A blockers. The significant increase in blood neutrophil count in the netakimab group may reflect efflux of neutrophils from inflamed tissues. We therefore hypothesize that neutrophil count and neutrophil-to-lymphocyte ratio could serve as markers of therapeutic efficiency for IL-17A-blocking antibodies in the context of active inflammation.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
A Phase I/II Clinical Trial to evaluate the efficacy of baricitinib to prevent respiratory insufficiency progression in onco-hematological patients affected with COVID19: A structured summary of a study protocol for a randomised controlled trial.Trials. 2021 Feb 5;22(1):116. doi: 10.1186/s13063-021-05072-4. Trials. 2021. PMID: 33546739 Free PMC article.
-
Baricitinib versus tocilizumab in critically ill COVID-19 patients: A retrospective cohort study.Pharmacotherapy. 2024 Jan;44(1):28-38. doi: 10.1002/phar.2867. Epub 2023 Aug 24. Pharmacotherapy. 2024. PMID: 37593883 Free PMC article.
-
Comparative Effectiveness of Baricitinib Versus Tocilizumab in Hospitalized Patients With COVID-19: A Retrospective Cohort Study of the National Covid Collaborative.Crit Care Med. 2025 Jan 1;53(1):e29-e41. doi: 10.1097/CCM.0000000000006444. Epub 2024 Oct 4. Crit Care Med. 2025. PMID: 39365115
-
Efficacy and safety of baricitinib and tocilizumab in hospitalized patients with COVID-19: A comparison using systematic review and meta-analysis.Front Pharmacol. 2022 Oct 14;13:1004308. doi: 10.3389/fphar.2022.1004308. eCollection 2022. Front Pharmacol. 2022. PMID: 36330085 Free PMC article.
-
Baricitinib combination therapy: a narrative review of repurposed Janus kinase inhibitor against severe SARS-CoV-2 infection.Infection. 2022 Apr;50(2):295-308. doi: 10.1007/s15010-021-01730-6. Epub 2021 Dec 13. Infection. 2022. PMID: 34902115 Free PMC article. Review.
Cited by
-
Coronavirus disease 2019 and cardiovascular disease.Tzu Chi Med J. 2023 Jun 13;35(3):213-220. doi: 10.4103/tcmj.tcmj_219_22. eCollection 2023 Jul-Sep. Tzu Chi Med J. 2023. PMID: 37545802 Free PMC article.
-
CCR5/CXCR3 antagonist TAK-779 prevents diffuse alveolar damage of the lung in the murine model of the acute respiratory distress syndrome.Front Pharmacol. 2024 Feb 21;15:1351655. doi: 10.3389/fphar.2024.1351655. eCollection 2024. Front Pharmacol. 2024. PMID: 38449806 Free PMC article.
-
The effects of combination-therapy of tocilizumab and baricitinib on the management of severe COVID-19 cases: a randomized open-label clinical trial.Front Pharmacol. 2023 Oct 19;14:1265541. doi: 10.3389/fphar.2023.1265541. eCollection 2023. Front Pharmacol. 2023. PMID: 37927607 Free PMC article.
-
Efficacy and safety of Ixekizumab vs. low-dose IL-2 vs. Colchicine vs. standard of care in the treatment of patients hospitalized with moderate-to-critical COVID-19: A pilot randomized clinical trial (STRUCK: Survival Trial Using Cytokine Inhibitors).Rev Soc Bras Med Trop. 2023 Apr 14;56:e0565. doi: 10.1590/0037-8682-0565-2022. eCollection 2023. Rev Soc Bras Med Trop. 2023. PMID: 37075454 Free PMC article. Clinical Trial.
-
COVID-19 Biomarkers at the Crossroad between Patient Stratification and Targeted Therapy: The Role of Validated and Proposed Parameters.Int J Mol Sci. 2023 Apr 12;24(8):7099. doi: 10.3390/ijms24087099. Int J Mol Sci. 2023. PMID: 37108262 Free PMC article. Review.
References
-
- Leisman DE, Ronner L, Pinotti R, Taylor MD, Sinha P, Calfee CS, et al.. Cytokine elevation in severe and critical COVID-19: a rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir Med. 2020; 8(12):1233–1244. doi: 10.1016/S2213-2600(20)30404-5 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

