Knockdown of CD146 promotes endothelial-to-mesenchymal transition via Wnt/β-catenin pathway
- PMID: 36001597
- PMCID: PMC9401105
- DOI: 10.1371/journal.pone.0273542
Knockdown of CD146 promotes endothelial-to-mesenchymal transition via Wnt/β-catenin pathway
Abstract
Purpose: Cardiac fibrosis is characterized by the excessive deposition of extracellular matrix (ECM) proteins and leads to the maladaptive changes in myocardium. Endothelial cells (ECs) undergoing mesenchymal transition contributes to the occurrence and development of cardiac fibrosis. CD146 is an adhesion molecule highly expressed in ECs. The present study was performed to explore the role of CD146 in modulating endothelial to mesenchymal transition (EndMT).
Methods: C57BL/6 mice were subjected to subcutaneous implantation of osmotic minipump infused with angiotensin II (Ang Ⅱ). Adenovirus carrying CD146 short hairpin RNA (shRNA) or CD146 encoding sequence were infected into cultured human umbilical vein endothelial cells (HUVECs) followed by stimulation with Ang II or transforming growth factor-β1 (TGF-β1). Differentially expressed genes were revealed by RNA-sequencing (RNA-Seq) analysis. Gene expression was measured by quantitative real-time PCR, and protein expression and distribution were determined by Western blot and immunofluorescence staining, respectively.
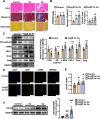
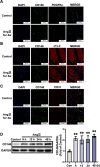
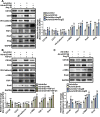
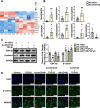
Results: CD146 was predominantly expressed by ECs in normal mouse hearts. CD146 was upregulated in ECs but not fibroblasts and myocytes in hearts of Ang II-infused mice and in HUVECs stimulated with Ang Ⅱ. RNA-Seq analysis revealed the differentially expressed genes related to EndMT and Wnt/β-catenin signaling pathway. CD146 knockdown and overexpression facilitated and attenuated, respectively, EndMT induced by Ang II or TGF-β1. CD146 knockdown upregulated Wnt pathway-related genes including Wnt4, LEF1, HNF4A, FOXA1, SOX6, and CCND3, and increased the protein level and nuclear translocation of β-catenin.
Conclusions: Knockdown of CD146 exerts promotional effects on EndMT via activating Wnt/β-catenin pathway and the upregulation of CD146 might play a protective role against EndMT and cardiac fibrosis.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

