The Abdominal Pain Unit (APU). Study protocol of a standardized and structured care pathway for patients with atraumatic abdominal pain in the emergency department: A stepped wedged cluster randomized controlled trial
- PMID: 36001620
- PMCID: PMC9401147
- DOI: 10.1371/journal.pone.0273115
The Abdominal Pain Unit (APU). Study protocol of a standardized and structured care pathway for patients with atraumatic abdominal pain in the emergency department: A stepped wedged cluster randomized controlled trial
Abstract
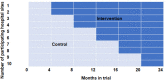
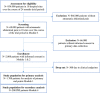
This study aims to improve emergency department (ED) care for patients suffering from atraumatic abdominal pain. An application-supported pathway for the ED will be implemented, which supports quick, evidence-based, and standardized diagnosis and treatment steps for patients with atraumatic abdominal pain at the ED. A mixed-methods multicentre cluster randomized controlled stepped wedge trial design will be applied. A total of 10 hospitals with EDs (expected n = 2.000 atraumatic abdominal pain patients) will consecutively (every 4 months) be randomized to apply the intervention. Inclusion criteria for patients are a minimum age of 18 years, suffering from atraumatic abdominal pain and being insured with a German statutory health insurance. Primary outcomes: acute pain score at time of discharge from ED, duration of treatment at the ED, patient-reported satisfaction. Secondary endpoints include patient safety and quality of care parameters, process evaluation parameters, and costs and cost-effectiveness parameters. Quantitative data will be gathered from patient-surveys, clinical records, and routine data from hospital information systems as well as from a participating German statutory health insurance. Descriptive and analytic statistical analysis will be performed to provide summaries and associations for primary patient-reported outcomes, process measures, quality measures, and costs. Qualitative data collection consists of participatory patient observations and semi-structured expert interviews, which will be inductively analysed. Findings will be disseminated in publications in peer-reviewed journals, on conferences, as well as via a project website. To ensure data protection, appropriate technical and organisational measures will be taken. Trial registration: DRKS00021052.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Mockel M, Searle J, Muller R, Slagman A, Storchmann H, Oestereich P, et al.. Chief complaints in medical emergencies: do they relate to underlying disease and outcome? The Charité Emergency Medicine Study (CHARITEM). European journal of emergency medicine: official journal of the European Society for Emergency Medicine. 2013;20(2):103–8. - PubMed
-
- Fagerström A, Paajanen P, Saarelainen H, Ahonen-Siirtola M, Ukkonen M, Miettinen P, et al.. Non-specific abdominal pain remains as the most common reason for acute abdomen: 26-year retrospective audit in one emergency unit. Scandinavian journal of gastroenterology. 2017;52(10):1072–7. doi: 10.1080/00365521.2017.1342140 - DOI - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources