A genetically engineered Plasmodium falciparum parasite vaccine provides protection from controlled human malaria infection
- PMID: 36001680
- PMCID: PMC10423335
- DOI: 10.1126/scitranslmed.abn9709
A genetically engineered Plasmodium falciparum parasite vaccine provides protection from controlled human malaria infection
Abstract
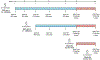
Genetically engineered live Plasmodium falciparum sporozoites constitute a potential platform for creating consistently attenuated, genetically defined, whole-parasite vaccines against malaria through targeted gene deletions. Such genetically attenuated parasites (GAPs) do not require attenuation by irradiation or concomitant drug treatment. We previously developed a P. falciparum (Pf) GAP with deletions in P52, P36, and SAP1 genes (PfGAP3KO) and demonstrated its safety and immunogenicity in humans. Here, we further assessed safety, tolerability, and immunogenicity of the PfGAP3KO vaccine and tested its efficacy against controlled human malaria infection (CHMI) in malaria-naïve subjects. The vaccine was delivered by three (n = 6) or five (n = 8) immunizations with ~200 PfGAP3KO-infected mosquito bites per immunization. PfGAP3KO was safe and well tolerated with no breakthrough P. falciparum blood stage infections. Vaccine-related adverse events were predominately localized urticaria related to the numerous mosquito bites administered per vaccination. CHMI via bites with mosquitoes carrying fully infectious Pf NF54 parasites was carried out 1 month after the last immunization. Half of the study participants who received either three or five PfGAP3KO immunizations remained P. falciparum blood stage negative, as shown by a lack of detection of Plasmodium 18S rRNA in the blood for 28 days after CHMI. Six protected study participants received a second CHMI 6 months later, and one remained completely protected. Thus, the PfGAP3KO vaccine was safe and immunogenic and was capable of inducing protection against sporozoite infection. These results warrant further evaluation of PfGAP3KO vaccine efficacy in dose-range finding trials with an injectable formulation.
Conflict of interest statement
Figures


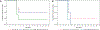

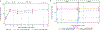
References
-
- World Malaria Report 2021 (2021) https://www.who.int/teams/global-malaria-programme/reports/world-malaria...).
-
- Kappe SH, Vaughan AM, Boddey JA, Cowman AF, That was then but this is now: Malaria research in the time of an eradication agenda. Science 328, 862–866 (2010). - PubMed
-
- RTS,S Clinical Trials Partnership [Agnandji, Lell B, Fernandes JF, Abossolo BP, Methogo BG, Kabwende AL, Adegnika AA, Mordmuller B, Issifou S, Kremsner PG, Sacarlal J, Aide P, Lanaspa M, Aponte JJ, Machevo S, Acacio S, Bulo H, Sigauque B, Macete E, Alonso P, Abdulla S, Salim N, Minja R, Mpina M, Ahmed S, Ali AM, Mtoro AT, Hamad AS, Mutani P, Tanner M, Tinto H, D’Alessandro U, Sorgho H, Valea I, Bihoun B, Guiraud I, Kabore B, Sombie O, Guiguemde RT, Ouedraogo JB, Hamel MJ, Kariuki S, Oneko M, Odero C, Otieno K, Awino N, McMorrow M, Muturi-Kioi V, Laserson KF, Slutsker L, Otieno W, Otieno L, Otsyula N, Gondi S, Otieno A, Owira V, Oguk E, Odongo G, Woods JB, Ogutu B, Njuguna P, Chilengi R, Akoo P, Kerubo C, Maingi C, Lang T, Olotu A, Bejon P, Marsh K, Mwambingu G, Owusu-Agyei S, Asante KP, Osei-Kwakye K, Boahen O, Dosoo D, Asante I, Adjei G, Kwara E, Chandramohan D, Greenwood B, Lusingu J, Gesase S, Malabeja A, Abdul O, Mahende C, Liheluka E, Malle L, Lemnge M, Theander TG, Drakeley C, Ansong D, Agbenyega T, Adjei S, Boateng HO, Rettig T, Bawa J, Sylverken J, Sambian D, Sarfo A, Agyekum A, Martinson F, Hoffman I, Mvalo T, Kamthunzi P, Nkomo R, Tembo T, Tegha G, Tsidya M, Kilembe J, Chawinga C, Ballou WR, Cohen J, Guerra Y, Jongert E, Lapierre D, Leach A, Lievens M, Ofori-Anyinam O, Olivier A, Vekemans J, Carter T, Kaslow D, Leboulleux D, Loucq C, Radford A, Savarese B, Schellenberg D, Sillman M, Vansadia P, A phase 3 trial of RTS,S/AS01 malaria vaccine in African infants. N Engl J Med 367, 2284–2295 (2012). - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

