Astrocyte calcium dysfunction causes early network hyperactivity in Alzheimer's disease
- PMID: 36001964
- PMCID: PMC9433881
- DOI: 10.1016/j.celrep.2022.111280
Astrocyte calcium dysfunction causes early network hyperactivity in Alzheimer's disease
Abstract
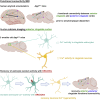
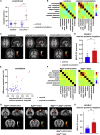
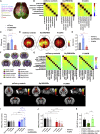
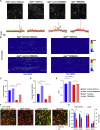
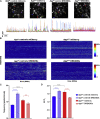
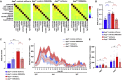
Dysfunctions of network activity and functional connectivity (FC) represent early events in Alzheimer's disease (AD), but the underlying mechanisms remain unclear. Astrocytes regulate local neuronal activity in the healthy brain, but their involvement in early network hyperactivity in AD is unknown. We show increased FC in the human cingulate cortex several years before amyloid deposition. We find the same early cingulate FC disruption and neuronal hyperactivity in AppNL-F mice. Crucially, these network disruptions are accompanied by decreased astrocyte calcium signaling. Recovery of astrocytic calcium activity normalizes neuronal hyperactivity and FC, as well as seizure susceptibility and day/night behavioral disruptions. In conclusion, we show that astrocytes mediate initial features of AD and drive clinically relevant phenotypes.
Keywords: App mice; BOLD rsfMRI; CP: Neuroscience; amyloid pathology; astrocytes; calcium signaling; neuronal hyperactivity.
Copyright © 2022. Published by Elsevier Inc.
Conflict of interest statement
Declaration of interests B.D.S. is the Bax-Vanluffelen Chair for Alzheimer’s Disease and is supported by the Opening the Future campaign and Mission Lucidity of KU-Leuven. D.R.T. received speaker honorarium from Biogen (USA), and collaborated with GE-Healthcare (UK), Novartis Pharma Basel (Switzerland), Probiodrug (Germany), and Janssen Pharmaceutical Companies (Belgium).
Figures
References
-
- Adamczuk K., De Weer A.S., Nelissen N., Chen K., Sleegers K., Bettens K., Van Broeckhoven C., Vandenbulcke M., Thiyyagura P., Dupont P., et al. Polymorphism of brain derived neurotrophic factor influences β amyloid load in cognitively intact apolipoprotein e ε4 carriers. Neuroimage. Clin. 2013;2:512–520. doi: 10.1016/j.nicl.2013.04.001. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

