Heterotropic roles of divalent cations in the establishment of allostery and affinity maturation of integrin αXβ2
- PMID: 36001965
- PMCID: PMC9440770
- DOI: 10.1016/j.celrep.2022.111254
Heterotropic roles of divalent cations in the establishment of allostery and affinity maturation of integrin αXβ2
Abstract
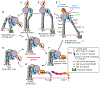
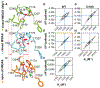
Allosteric activation and silencing of leukocyte β2-integrins transpire through cation-dependent structural changes, which mediate integrin biosynthesis and recycling, and are essential to designing leukocyte-specific drugs. Stepwise addition of Mg2+ reveals two mutually coupled events for the αXβ2 ligand-binding domain-the αX I-domain-corresponding to allostery establishment and affinity maturation. Electrostatic alterations in the Mg2+-binding site establish long-range couplings, leading to both pH- and Mg2+-occupancy-dependent biphasic stability change in the αX I-domain fold. The ligand-binding sensorgrams show composite affinity events for the αX I-domain accounting for the multiplicity of the αX I-domain conformational states existing in the solution. On cell surfaces, increasing Mg2+ concentration enhanced adhesiveness of αXβ2. This work highlights how intrinsically flexible pH- and cation-sensitive architecture endows a unique dynamic continuum to the αI-domain structure on the intact integrin, thereby revealing the importance of allostery establishment and affinity maturation in both extracellular and intracellular integrin events.
Keywords: CD11c; CD18; CP: Molecular biology; affinity maturation; allostery; integrin; αX I-domain.
Copyright © 2022 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures
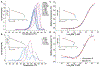



Similar articles
-
Characterization of alphaX I-domain binding to Thy-1.Biochem Biophys Res Commun. 2005 Jun 3;331(2):557-61. doi: 10.1016/j.bbrc.2005.04.006. Biochem Biophys Res Commun. 2005. PMID: 15850796
-
Identification of critical residues for plasminogen binding by the alphaX I-domain of the beta2 integrin, alphaXbeta2.Mol Cells. 2007 Oct 31;24(2):240-6. Mol Cells. 2007. PMID: 17978577
-
Characterization of αX I-Domain Binding to Receptors for Advanced Glycation End Products (RAGE).Mol Cells. 2017 May 31;40(5):355-362. doi: 10.14348/molcells.2017.0021. Epub 2017 May 24. Mol Cells. 2017. PMID: 28535664 Free PMC article.
-
Conformational regulation of integrin structure and function.Annu Rev Biophys Biomol Struct. 2002;31:485-516. doi: 10.1146/annurev.biophys.31.101101.140922. Epub 2001 Oct 25. Annu Rev Biophys Biomol Struct. 2002. PMID: 11988479 Review.
-
The regulation of integrin function by divalent cations.Cell Adh Migr. 2012 Jan-Feb;6(1):20-9. doi: 10.4161/cam.18702. Cell Adh Migr. 2012. PMID: 22647937 Free PMC article. Review.
Cited by
-
Expression and Characterization of Intein-Cyclized Trimer of Staphylococcus aureus Protein A Domain Z.Int J Mol Sci. 2023 Jan 9;24(2):1281. doi: 10.3390/ijms24021281. Int J Mol Sci. 2023. PMID: 36674796 Free PMC article.
References
-
- Azcutia V, Routledge M, Williams MR, Newton G, Frazier WA, Manica A, Croce KJ, Parkos CA, Schmider AB, Turman MV, et al. (2013). CD47 plays a critical role in T-cell recruitment by regulation of LFA-1 and VLA-4 integrin adhesive functions. Mol. Biol. Cell 24, 3358–3368. 10.1091/mbc.E13-01-0063. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

