Outcomes with adjuvant anti-PD-1 therapy in patients with sentinel lymph node-positive melanoma without completion lymph node dissection
- PMID: 36002183
- PMCID: PMC9413295
- DOI: 10.1136/jitc-2021-004417
Outcomes with adjuvant anti-PD-1 therapy in patients with sentinel lymph node-positive melanoma without completion lymph node dissection
Abstract
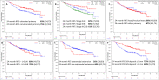
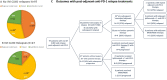
Until recently, most patients with sentinel lymph node-positive (SLN+) melanoma underwent a completion lymph node dissection (CLND), as mandated in published trials of adjuvant systemic therapies. Following multicenter selective lymphadenectomy trial-II, most patients with SLN+ melanoma no longer undergo a CLND prior to adjuvant systemic therapy. A retrospective analysis of clinical outcomes in SLN+ melanoma patients treated with adjuvant systemic therapy after July 2017 was performed in 21 international cancer centers. Of 462 patients who received systemic adjuvant therapy, 326 patients received adjuvant anti-PD-1 without prior immediate (IM) CLND, while 60 underwent IM CLND. With median follow-up of 21 months, 24-month relapse-free survival (RFS) was 67% (95% CI 62% to 73%) in the 326 patients. When the patient subgroups who would have been eligible for the two adjuvant anti-PD-1 clinical trials mandating IM CLND were analyzed separately, 24-month RFS rates were 64%, very similar to the RFS rates from those studies. Of these no-CLND patients, those with SLN tumor deposit >1 mm, stage IIIC/D and ulcerated primary had worse RFS. Of the patients who relapsed on adjuvant anti-PD-1, those without IM CLND had a higher rate of relapse in the regional nodal basin than those with IM CLND (46% vs 11%). Therefore, 55% of patients who relapsed without prior CLND underwent surgery including therapeutic lymph node dissection (TLND), with 30% relapsing a second time; there was no difference in subsequent relapse between patients who received observation vs secondary adjuvant therapy. Despite the increased frequency of nodal relapses, adjuvant anti-PD-1 therapy may be as effective in SLN+ pts who forego IM CLND and salvage surgery with TLND at relapse may be a viable option for these patients.
Keywords: Adjuvants, Immunologic; Melanoma.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: ZE: Advisory boards: Array, Pfizer, OncoSec, Regeneron, Genentech, Novartis, Natera. Research funding: Novartis, Pfizer, Boehringer-Ingelheim. JFT: Advisory boards: BMS Australia, MSD Australia, GSK, Provectus Inc. Travel support: GSK, Provectus Inc and Novartis. TJH: Research funding: Genentech, SkylineDX. EKB: Research funding: SkylineDx, Honorarium: Excite International inc. DEG: Honoraria: BMS, Novartis, Q biotics. GK; Advisory Board: Merck. AVA: Advisory Boards: Amgen, Bristol-Myers Squibb, Novartis, MSD-Merck, Merck-Pfizer, Pierre Fabre, Provectus, Sanofi, Sirius Medical, 4SC Research funding: Amgen, Merck-Pfizer. JV: Speaker: Caste Biosciences. GB: Research funding: Istari Oncology, Delcath, Oncosec Medical, Replimune, Checkmate Pharmaceuticals. ROB: Advisory boards: Amgen, BD/BARD, Bristol-Myers Squibb, Merck Sharp & Dohme, Novartis, Roche and Sanofi Genzyme. Research funding: Bristol-Myers Squibb, SkyLineDx. Speaker honorarium: Roche and Pfizer. Shareholder in SATMEG Ventures AB. NIK: Advisory boards: Bristol-Myers Squibb, AstraZeneca, Regeneron, Array, Immunocore, Merck, Incyte, Jounce Therapeutics,Pfizer,Novartis, Nektar,Castle Biosciences, Instil Bio. Research funding: Bristol-Myers Squibb, Merck, Novartis, Celgene, Replimune, Amgen, Regneron, HUYA, GlaxoSmithKline Stocks: Bellicum Pharmaceuticals, Amarin Corporation, Asensus Surgical.
Figures

References
-
- Ascierto PA, Del Vecchio M, Mandalá M, et al. . Adjuvant nivolumab versus ipilimumab in resected stage IIIB-C and stage IV melanoma (CheckMate 238): 4-year results from a multicentre, double-blind, randomised, controlled, phase 3 trial. Lancet Oncol 2020;21:1465–77. 10.1016/S1470-2045(20)30494-0 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
