Adult Born Dentate Granule Cell Mediated Upregulation of Feedback Inhibition in a Mouse Model of Traumatic Brain Injury
- PMID: 36002261
- PMCID: PMC9480876
- DOI: 10.1523/JNEUROSCI.2263-21.2022
Adult Born Dentate Granule Cell Mediated Upregulation of Feedback Inhibition in a Mouse Model of Traumatic Brain Injury
Abstract
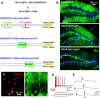
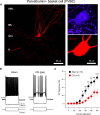
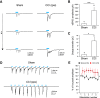
Post-traumatic epilepsy (PTE) and behavioral comorbidities frequently develop after traumatic brain injury (TBI). Aberrant neurogenesis of dentate granule cells (DGCs) after TBI may contribute to the synaptic reorganization that occurs in PTE, but how neurogenesis at different times relative to the injury contributes to feedback inhibition and recurrent excitation in the dentate gyrus is unknown. Thus, we examined whether DGCs born at different postnatal ages differentially participate in feedback inhibition and recurrent excitation in the dentate gyrus using the controlled cortical impact (CCI) model of TBI. Both sexes of transgenic mice expressing channelrhodopsin2 (ChR2) in postnatally born DGCs were used for optogenetic activation of three DGC cohorts: postnatally early born DGCs, or those born just before or after CCI. We performed whole-cell patch-clamp recordings from ChR2-negative, mature DGCs and parvalbumin-expressing basket cells (PVBCs) in hippocampal slices to determine whether optogenetic activation of postnatally born DGCs increases feedback inhibition and/or recurrent excitation in mice 8-10 weeks after CCI and whether PVBCs are targets of ChR2-positive DGCs. In the dentate gyrus ipsilateral to CCI, activation of ChR2-expressing DGCs born before CCI produced increased feedback inhibition in ChR2-negative DGCs and increased excitation in PVBCs compared with those from sham controls. This upregulated feedback inhibition was less prominent in DGCs born early in life or after CCI. Surprisingly, ChR2-positive DGC activation rarely evoked recurrent excitation in mature DGCs from any cohort. These results support that DGC birth date-related increased feedback inhibition in of DGCs may contribute to altered excitability after TBI.SIGNIFICANCE STATEMENT Dentate granule cells (DGCs) control excitability of the dentate gyrus through synaptic interactions with inhibitory GABAergic interneurons. Persistent changes in DGC synaptic connectivity develop after traumatic brain injury, contributing to hyperexcitability in post-traumatic epilepsy (PTE). However, the impact of DGC neurogenesis on synaptic reorganization, especially on inhibitory circuits, after brain injury is not adequately described. Here, upregulation of feedback inhibition in mature DGCs from male and female mice was associated with increased excitation of parvalbumin-expressing basket cells by postnatally born DGCs, providing novel insights into underlying mechanisms of altered excitability after brain injury. A better understanding of these inhibitory circuit changes can help formulate hypotheses for development of novel, evidence-based treatments for post-traumatic epilepsy by targeting birth date-specific subsets of DGCs.
Keywords: adult neurogenesis; mossy fiber sprouting; optogenetics; parvalbumin-expressing basket cell; patch-clamp; post-traumatic epilepsy.
Copyright © 2022 the authors.
Figures






Comment in
-
Adult-Born Granule Cells Contribute to Dentate Gyrus Circuit Reorganization after Traumatic Brain Injury.J Neurosci. 2023 Feb 8;43(6):879-881. doi: 10.1523/JNEUROSCI.1994-22.2022. J Neurosci. 2023. PMID: 36754637 Free PMC article. No abstract available.
-
Adult-Generated Dentate Granule Cells in Epilepsy: Loyal Gatekeepers or Evil Changelings?Epilepsy Curr. 2022 Dec 22;23(2):115-117. doi: 10.1177/15357597221145262. eCollection 2023 Mar-Apr. Epilepsy Curr. 2022. PMID: 37122409 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
