A neural correlate of perceptual segmentation in macaque middle temporal cortical area
- PMID: 36002445
- PMCID: PMC9402536
- DOI: 10.1038/s41467-022-32555-y
A neural correlate of perceptual segmentation in macaque middle temporal cortical area
Abstract
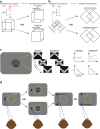
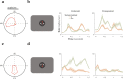
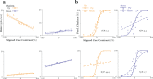
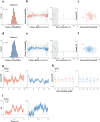
High-resolution vision requires fine retinal sampling followed by integration to recover object properties. Importantly, accuracy is lost if local samples from different objects are intermixed. Thus, segmentation, grouping of image regions for separate processing, is crucial for perception. Previous work has used bi-stable plaid patterns, which can be perceived as either a single or multiple moving surfaces, to study this process. Here, we report a relationship between activity in a mid-level site in the primate visual pathways and segmentation judgments. Specifically, we find that direction selective middle temporal neurons are sensitive to texturing cues used to bias the perception of bi-stable plaids and exhibit a significant trial-by-trial correlation with subjective perception of a constant stimulus. This correlation is greater in units that signal global motion in patterns with multiple local orientations. Thus, we conclude the middle temporal area contains a signal for segmenting complex scenes into constituent objects and surfaces.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

