Translational feasibility and efficacy of nasal photodynamic disinfection of SARS-CoV-2
- PMID: 36002557
- PMCID: PMC9400568
- DOI: 10.1038/s41598-022-18513-0
Translational feasibility and efficacy of nasal photodynamic disinfection of SARS-CoV-2
Abstract
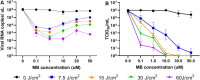
The lack of therapeutic options to fight Covid-19 has contributed to the current global pandemic. Despite the emergence of effective vaccines, development of broad-spectrum antiviral treatment remains a significant challenge, in which antimicrobial photodynamic therapy (aPDT) may play a role, especially at early stages of infection. aPDT of the nares with methylene blue (MB) and non-thermal light has been successfully utilized to inactivate both bacterial and viral pathogens in the perioperative setting. Here, we investigated the effect of MB-aPDT to inactivate human betacoronavirus OC43 and SARS-CoV-2 in vitro and in a proof-of-principle COVID-19 clinical trial to test, in a variety of settings, the practicality, technical feasibility, and short-term efficacy of the method. aPDT yielded inactivation of up to 6-Logs in vitro, as measured by RT-qPCR and infectivity assay. From a photo-physics perspective, the in vitro results suggest that the response is not dependent on the virus itself, motivating potential use of aPDT for local destruction of SARS-CoV-2 and its variants. In the clinical trial we observed variable effects on viral RNA in nasal-swab samples as assessed by RT-qPCR attributed to aPDT-induced RNA fragmentation causing falsely-elevated counts. However, the viral infectivity in clinical nares swabs was reduced in 90% of samples and undetectable in 70% of samples. This is the first demonstration based on quantitative clinical viral infectivity measurements that MB-aPDT is a safe, easily delivered and effective front-line technique that can reduce local SARS-CoV-2 viral load.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


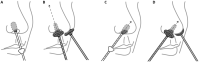
Similar articles
-
Evaluation of methylene blue based photodynamic inactivation (PDI) against intracellular B-CoV and SARS-CoV2 viruses under different light sources in vitro as a basis for new local treatment strategies in the early phase of a Covid19 infection.Photodiagnosis Photodyn Ther. 2022 Mar;37:102642. doi: 10.1016/j.pdpdt.2021.102642. Epub 2021 Dec 2. Photodiagnosis Photodyn Ther. 2022. PMID: 34863949 Free PMC article.
-
Photodynamic disinfection of SARS-CoV-2 clinical samples using a methylene blue formulation.Photochem Photobiol Sci. 2022 Jun;21(6):1101-1109. doi: 10.1007/s43630-022-00202-6. Epub 2022 Mar 19. Photochem Photobiol Sci. 2022. PMID: 35304729 Free PMC article.
-
aPDT for oral decontamination of hospitalized patients with COVID 19.Photodiagnosis Photodyn Ther. 2022 Jun;38:102762. doi: 10.1016/j.pdpdt.2022.102762. Epub 2022 Feb 16. Photodiagnosis Photodyn Ther. 2022. PMID: 35181510 Free PMC article.
-
Nano Antiviral Photodynamic Therapy: a Probable Biophysicochemical Management Modality in SARS-CoV-2.Expert Opin Drug Deliv. 2021 Feb;18(2):265-272. doi: 10.1080/17425247.2021.1829591. Epub 2020 Oct 19. Expert Opin Drug Deliv. 2021. PMID: 33019838 Review.
-
Nanomaterials enabling clinical translation of antimicrobial photodynamic therapy.J Control Release. 2022 Jun;346:300-316. doi: 10.1016/j.jconrel.2022.04.035. Epub 2022 Apr 28. J Control Release. 2022. PMID: 35483636 Review.
Cited by
-
Femtosecond pulsed laser photodynamic therapy activates melanin and eradicates malignant melanoma.Proc Natl Acad Sci U S A. 2024 Apr 2;121(14):e2316303121. doi: 10.1073/pnas.2316303121. Epub 2024 Mar 29. Proc Natl Acad Sci U S A. 2024. PMID: 38551838 Free PMC article.
-
Dominant CT Patterns and Immune Responses during the Early Infection Phases of Different SARS-CoV-2 Variants.Viruses. 2023 May 31;15(6):1304. doi: 10.3390/v15061304. Viruses. 2023. PMID: 37376606 Free PMC article.
-
Light-based therapy of infected wounds: a review of dose considerations for photodynamic microbial inactivation and photobiomodulation.J Biomed Opt. 2025 Mar;30(3):030901. doi: 10.1117/1.JBO.30.3.030901. Epub 2025 Feb 7. J Biomed Opt. 2025. PMID: 39925694 Free PMC article. Review.
-
COVID-19 Incidence and Vaccine Effectiveness in University Staff, 1 March 2020-2 April 2022.Vaccines (Basel). 2023 Feb 19;11(2):483. doi: 10.3390/vaccines11020483. Vaccines (Basel). 2023. PMID: 36851360 Free PMC article.
-
Application of methylene blue for the prevention and treatment of COVID-19: A narrative review.Iran J Basic Med Sci. 2024;27(7):780-792. doi: 10.22038/IJBMS.2024.71871.15617. Iran J Basic Med Sci. 2024. PMID: 38800024 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

