RASA2 ablation in T cells boosts antigen sensitivity and long-term function
- PMID: 36002574
- PMCID: PMC9433322
- DOI: 10.1038/s41586-022-05126-w
RASA2 ablation in T cells boosts antigen sensitivity and long-term function
Abstract
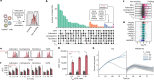
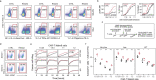
The efficacy of adoptive T cell therapies for cancer treatment can be limited by suppressive signals from both extrinsic factors and intrinsic inhibitory checkpoints1,2. Targeted gene editing has the potential to overcome these limitations and enhance T cell therapeutic function3-10. Here we performed multiple genome-wide CRISPR knock-out screens under different immunosuppressive conditions to identify genes that can be targeted to prevent T cell dysfunction. These screens converged on RASA2, a RAS GTPase-activating protein (RasGAP) that we identify as a signalling checkpoint in human T cells, which is downregulated upon acute T cell receptor stimulation and can increase gradually with chronic antigen exposure. RASA2 ablation enhanced MAPK signalling and chimeric antigen receptor (CAR) T cell cytolytic activity in response to target antigen. Repeated tumour antigen stimulations in vitro revealed that RASA2-deficient T cells show increased activation, cytokine production and metabolic activity compared with control cells, and show a marked advantage in persistent cancer cell killing. RASA2-knockout CAR T cells had a competitive fitness advantage over control cells in the bone marrow in a mouse model of leukaemia. Ablation of RASA2 in multiple preclinical models of T cell receptor and CAR T cell therapies prolonged survival in mice xenografted with either liquid or solid tumours. Together, our findings highlight RASA2 as a promising target to enhance both persistence and effector function in T cell therapies for cancer treatment.
© 2022. The Author(s).
Conflict of interest statement
J.E. is a compensated co-founder at Mnemo Therapeutics, a compensated scientific advisor to Cytovia Therapeutics, owns stocks in Mnemo Therapeutica and Cytovia Therapeutics and has received a consulting fee from Casdin Capital. The Eyquem laboratory has received research support from Cytovia Therapeutic and Takeda. J.E. is a holder of patents pertaining to but not resulting from this work. A.A. is a co-founder of Tango Therapeutics, Azkarra Therapeutics, Ovibio Corporation and Kytarro, a member of the boards of Cytomx and Cambridge Science Corporation, a member of the scientific advisory boards of Genentech, GLAdiator, Circle, Bluestar, Earli, Ambagon, Phoenix Molecular Designs and Trial Library, a consultant for SPARC, ProLynx and GSK, and a recipient of grant or research support from SPARC and AstraZeneca, and holds patents on the use of PARP inhibitors held jointly with AstraZeneca from which he has benefited financially (and may do so in the future). A.M. is a cofounder of Arsenal Biosciences, Spotlight Therapeutics and Survey Genomics, serves on the boards of directors at Spotlight Therapeutics and Survey Genomics, is board observer (and former member of the board of directors) at Arsenal Biosciences, is a member of the scientific advisory boards of Arsenal Biosciences, Spotlight Therapeutics, Survey Genomics and NewLimit, owns stock in Arsenal Biosciences, Spotlight Therapeutics, NewLimit, Survey Genomics, PACT Pharma, and Merck, and has received fees from Arsenal Biosciences, Spotlight Therapeutics, NewLimit, 23andMe, PACT Pharma, Juno Therapeutics, Trizell, Vertex, Merck, Amgen, Genentech, AlphaSights, Rupert Case Management, Bernstein and ALDA. A.M. is an investor in and informal advisor to Offline Ventures and a client of EPIQ. The Marson laboratory has received research support from Juno Therapeutics, Epinomics, Sanofi, GlaxoSmithKline, Gilead and Anthem. S.G. has a consulting agreement with Tessa Therapeutics, and is a compensated DSMB member of Immatics, and has received honoraria from Tidal, Catamaran Bio and Novartis within the last 2 years. The Gottschalk laboratory has received research support from Tessa Therapeutics. A.T.S. is a scientific founder of Immunai and founder of Cartography Biosciences and receives research funding from Arsenal Biosciences, Allogene Therapeutics and Merck Research Laboratories. H.O. has been a compensated consultant within the last 2 years for Bristol-Myers Squibb, Alexion Pharmaceuticals, Amal Therapeutics, Servier Pharmaceuticals, Neuvogen and Eureka Therapeutics. J.C., E.S., A.A. and A.M. are listed as inventors on a world patent application (WO2020014235A1) related to this work,
Figures












Comment in
-
RASA2 a new gatekeeper for TCR signaling.Am J Transplant. 2022 Dec;22(12):2715. doi: 10.1111/ajt.16684. Am J Transplant. 2022. PMID: 36458703 No abstract available.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

