Type I IFNs promote cancer cell stemness by triggering the epigenetic regulator KDM1B
- PMID: 36002648
- PMCID: PMC9477743
- DOI: 10.1038/s41590-022-01290-3
Type I IFNs promote cancer cell stemness by triggering the epigenetic regulator KDM1B
Erratum in
-
Author Correction: Type I IFNs promote cancer cell stemness by triggering the epigenetic regulator KDM1B.Nat Immunol. 2024 Nov;25(11):2166. doi: 10.1038/s41590-024-01984-w. Nat Immunol. 2024. PMID: 39289560 Free PMC article. No abstract available.
Abstract
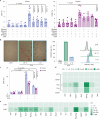
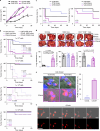
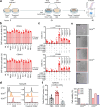
Cancer stem cells (CSCs) are a subpopulation of cancer cells endowed with high tumorigenic, chemoresistant and metastatic potential. Nongenetic mechanisms of acquired resistance are increasingly being discovered, but molecular insights into the evolutionary process of CSCs are limited. Here, we show that type I interferons (IFNs-I) function as molecular hubs of resistance during immunogenic chemotherapy, triggering the epigenetic regulator demethylase 1B (KDM1B) to promote an adaptive, yet reversible, transcriptional rewiring of cancer cells towards stemness and immune escape. Accordingly, KDM1B inhibition prevents the appearance of IFN-I-induced CSCs, both in vitro and in vivo. Notably, IFN-I-induced CSCs are heterogeneous in terms of multidrug resistance, plasticity, invasiveness and immunogenicity. Moreover, in breast cancer (BC) patients receiving anthracycline-based chemotherapy, KDM1B positively correlated with CSC signatures. Our study identifies an IFN-I → KDM1B axis as a potent engine of cancer cell reprogramming, supporting KDM1B targeting as an attractive adjunctive to immunogenic drugs to prevent CSC expansion and increase the long-term benefit of therapy.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures











Comment in
-
Immuno-epigenetic escape of cancer stem cells.Nat Immunol. 2022 Sep;23(9):1300-1302. doi: 10.1038/s41590-022-01293-0. Nat Immunol. 2022. PMID: 36002649 No abstract available.
-
Type I interferon induces cancer stem cells-mediated chemotherapy resistance.Oncoimmunology. 2022 Sep 22;11(1):2127274. doi: 10.1080/2162402X.2022.2127274. eCollection 2022. Oncoimmunology. 2022. PMID: 36185803 Free PMC article.
References
-
- Dean, M., Fojo, T. & Bates, S. Tumour stem cells and drug resistance. Nat. Rev. Cancer5, 275–284 (2005). - PubMed
-
- Batlle, E. & Clevers, H. Cancer stem cells revisited. Nat. Med.23, 1124–1134 (2017). - PubMed
-
- Vitale, I., Shema, E., Loi, S. & Galluzzi, L. Intratumoral heterogeneity in cancer progression and response to immunotherapy. Nat. Med.27, 212–224 (2021). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

