Mucosal immunology of the ocular surface
- PMID: 36002743
- PMCID: PMC9400566
- DOI: 10.1038/s41385-022-00551-6
Mucosal immunology of the ocular surface
Abstract
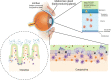
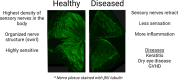
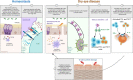
The eye is a sensory organ exposed to the environment and protected by a mucosal tissue barrier. While it shares a number of features with other mucosal tissues, the ocular mucosal system, composed of the conjunctiva, Meibomian glands, and lacrimal glands, is specialized to address the unique needs of (a) lubrication and (b) host defense of the ocular surface. Not surprisingly, most challenges, physical and immunological, to the homeostasis of the eye fall into those two categories. Dry eye, a dysfunction of the lacrimal glands and/or Meibomian glands, which can both cause, or arise from, sensory defects, including those caused by corneal herpes virus infection, serve as examples of these perturbations and will be discussed ahead. To preserve vision, dense neuronal and immune networks sense various stimuli and orchestrate responses, which must be tightly controlled to provide protection, while simultaneously minimizing collateral damage. All this happens against the backdrop of, and can be modified by, the microorganisms that colonize the ocular mucosa long term, or that are simply transient passengers introduced from the environment. This review will attempt to synthesize the existing knowledge and develop trends in the study of the unique mucosal and immune elements of the ocular surface.
© 2022. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply 2022.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Knop E, Knop N. Lacrimal drainage-associated lymphoid tissue (LDALT): a part of the human mucosal immune system. Invest Ophthalmol. Vis. Sci. 2001;42:566–574. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

