Induction, decay, and determinants of functional antibodies following vaccination with the RTS,S malaria vaccine in young children
- PMID: 36002841
- PMCID: PMC9402280
- DOI: 10.1186/s12916-022-02466-2
Induction, decay, and determinants of functional antibodies following vaccination with the RTS,S malaria vaccine in young children
Abstract
Background: RTS,S is the first malaria vaccine recommended for implementation among young children at risk. However, vaccine efficacy is modest and short-lived. Antibodies play the major role in vaccine-induced immunity, but knowledge on the induction, decay, and determinants of antibody function is limited, especially among children. Antibodies that promote opsonic phagocytosis and other cellular functions appear to be important contributors to RTS,S immunity.
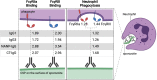
Methods: We studied a phase IIb trial of RTS,S/AS02 conducted in young children in malaria-endemic regions of Mozambique. We evaluated the induction of antibodies targeting the circumsporozoite protein (CSP, vaccine antigen) that interact with Fcγ-receptors (FcRγs) and promote phagocytosis (neutrophils, monocytes, THP-1 cells), antibody-dependent respiratory burst (ADRB) by neutrophils, and natural killer (NK) cell activity, as well as the temporal kinetics of responses over 5 years of follow-up (ClinicalTrials.gov registry number NCT00197041).
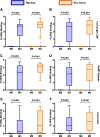
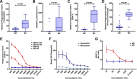
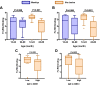
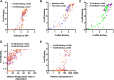
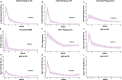
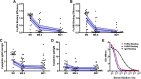
Results: RTS,S vaccination induced CSP-specific IgG with FcγRIIa and FcγRIII binding activity and promoted phagocytosis by neutrophils, THP-1 monocytes, and primary human monocytes, neutrophil ADRB activity, and NK cell activation. Responses were highly heterogenous among children, and the magnitude of neutrophil phagocytosis by antibodies was relatively modest, which may reflect modest vaccine efficacy. Induction of functional antibodies was lower among children with higher malaria exposure. Functional antibody magnitude and the functional activity of antibodies largely declined within a year post-vaccination, and decay were highest in the first 6 months, consistent with the decline in vaccine efficacy over that time. Decay rates varied for different antibody parameters and decay was slower for neutrophil phagocytosis. Biostatistical modelling suggested IgG1 and IgG3 contribute in promoting FcγR binding and phagocytosis, and IgG targeting the NANP-repeat and C-terminal regions CSP were similarly important for functional activities.
Conclusions: Results provide new insights to understand the modest and time-limited efficacy of RTS,S in children and the induction of antibody functional activities. Improving the induction and maintenance of antibodies that promote phagocytosis and cellular functions, and combating the negative effect of malaria exposure on vaccine responses are potential strategies for improving RTS,S efficacy and longevity.
Keywords: Antibodies; Children; Fcγ-receptor; Malaria; Monocytes; Neutrophils; Phagocytosis; Vaccines.
© 2022. The Author(s).
Conflict of interest statement
The authors have no conflicts of interest to declare. All co-authors have seen and agree with the contents of the manuscript and there is no financial interest to report.
Figures
Similar articles
-
Induction and decay of functional complement-fixing antibodies by the RTS,S malaria vaccine in children, and a negative impact of malaria exposure.BMC Med. 2019 Feb 25;17(1):45. doi: 10.1186/s12916-019-1277-x. BMC Med. 2019. PMID: 30798787 Free PMC article.
-
Antibody mechanisms of protection against malaria in RTS,S-vaccinated children: a post-hoc serological analysis of phase 2 trial.Lancet Microbe. 2024 Oct;5(10):100898. doi: 10.1016/S2666-5247(24)00130-7. Epub 2024 Aug 7. Lancet Microbe. 2024. PMID: 39127054 Clinical Trial.
-
Baseline exposure, antibody subclass, and hepatitis B response differentially affect malaria protective immunity following RTS,S/AS01E vaccination in African children.BMC Med. 2018 Oct 31;16(1):197. doi: 10.1186/s12916-018-1186-4. BMC Med. 2018. PMID: 30376866 Free PMC article. Clinical Trial.
-
Characterization of T-cell immune responses in clinical trials of the candidate RTS,S malaria vaccine.Hum Vaccin Immunother. 2018 Jan 2;14(1):17-27. doi: 10.1080/21645515.2017.1381809. Epub 2017 Dec 1. Hum Vaccin Immunother. 2018. PMID: 28934066 Free PMC article. Review.
-
The challenges of a circumsporozoite protein-based malaria vaccine.Expert Rev Vaccines. 2021 Feb;20(2):113-125. doi: 10.1080/14760584.2021.1874924. Epub 2021 Feb 7. Expert Rev Vaccines. 2021. PMID: 33554669 Review.
Cited by
-
Antibody-Dependent Respiratory Burst against Plasmodium falciparum Merozoites in Individuals Living in an Area with Declining Malaria Transmission.Vaccines (Basel). 2024 Feb 16;12(2):203. doi: 10.3390/vaccines12020203. Vaccines (Basel). 2024. PMID: 38400186 Free PMC article.
-
Induction of Fc-dependent functional antibodies against different variants of SARS-CoV-2 varies by vaccine type and prior infection.Commun Med (Lond). 2024 Dec 19;4(1):273. doi: 10.1038/s43856-024-00686-6. Commun Med (Lond). 2024. PMID: 39702507 Free PMC article.
-
Malaria vaccines: a new era of prevention and control.Nat Rev Microbiol. 2024 Dec;22(12):756-772. doi: 10.1038/s41579-024-01065-7. Epub 2024 Jul 18. Nat Rev Microbiol. 2024. PMID: 39025972 Review.
-
Malaria Vaccines: Progress to Date.BioDrugs. 2023 Nov;37(6):737-756. doi: 10.1007/s40259-023-00623-4. Epub 2023 Sep 20. BioDrugs. 2023. PMID: 37728713 Free PMC article. Review.
-
Precision adjuvants for pediatric vaccines.Sci Transl Med. 2024 Sep 4;16(763):eabq7378. doi: 10.1126/scitranslmed.abq7378. Epub 2024 Sep 4. Sci Transl Med. 2024. PMID: 39231242 Free PMC article. Review.
References
-
- World Health Organization . World Malaria Report 2021. Geneva: World Health Organization; 2021.
-
- World Health Organization . Malaria vaccine: WHO position paper - January 2016. 2016.
-
- World Health Organization . Ghana, Kenya and Malawi to take part in WHO malaria vaccine pilot programme. 2017.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

