Microglial TYROBP/DAP12 in Alzheimer's disease: Transduction of physiological and pathological signals across TREM2
- PMID: 36002854
- PMCID: PMC9404585
- DOI: 10.1186/s13024-022-00552-w
Microglial TYROBP/DAP12 in Alzheimer's disease: Transduction of physiological and pathological signals across TREM2
Abstract
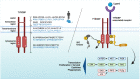
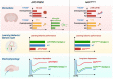
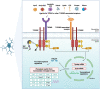
TYROBP (also known as DAP12 or KARAP) is a transmembrane adaptor protein initially described as a receptor-activating subunit component of natural killer (NK) cells. TYROBP is expressed in numerous cell types, including peripheral blood monocytes, macrophages, dendritic cells, and osteoclasts, but a key point of recent interest is related to the critical role played by TYROBP in the function of many receptors expressed on the plasma membrane of microglia. TYROBP is the downstream adaptor and putative signaling partner for several receptors implicated in Alzheimer's disease (AD), including SIRP1β, CD33, CR3, and TREM2. TYROBP has received much of its current notoriety because of its importance in brain homeostasis by signal transduction across those receptors. In this review, we provide an overview of evidence indicating that the biology of TYROBP extends beyond its interaction with these four ligand-binding ectodomain-intramembranous domain molecules. In addition to reviewing the structure and localization of TYROBP, we discuss our recent progress using mouse models of either cerebral amyloidosis or tauopathy that were engineered to be TYROBP-deficient or TYROBP-overexpressing. Remarkably, constitutively TYROBP-deficient mice provided a model of genetic resilience to either of the defining proteinopathies of AD. Learning behavior and synaptic electrophysiological function were preserved at normal physiological levels even in the face of robust cerebral amyloidosis (in APP/PSEN1;Tyrobp-/- mice) or tauopathy (in MAPTP301S;Tyrobp-/- mice). A fundamental underpinning of the functional synaptic dysfunction associated with each proteotype was an accumulation of complement C1q. TYROBP deficiency prevented C1q accumulation associated with either proteinopathy. Based on these data, we speculate that TYROBP plays a key role in the microglial sensome and the emergence of the disease-associated microglia (DAM) phenotype. TYROBP may also play a key role in the loss of markers of synaptic integrity (e.g., synaptophysin-like immunoreactivity) that has long been held to be the feature of human AD molecular neuropathology that most closely correlates with concurrent clinical cognitive function.
Keywords: Alzheimer; Amyloid; ApoE; Complement C1q; Disease-Associated Microglia (DAM); Sensome; Tau; Trem2; Tyrobp/Dap12; miR-155.
© 2022. The Author(s).
Conflict of interest statement
The authors declared no competing of interests.
Figures
References
-
- Um JW, Kaufman AC, Kostylev M, Heiss JK, Stagi M, Takahashi H, Kerrisk ME, Vortmeyer A, Wisniewski T, Koleske AJ, et al. Metabotropic glutamate receptor 5 is a coreceptor for Alzheimer abeta oligomer bound to cellular prion protein. Neuron. 2013;79:887–902. doi: 10.1016/j.neuron.2013.06.036. - DOI - PMC - PubMed
-
- Raka F, Di Sebastiano AR, Kulhawy SC, Ribeiro FM, Godin CM, Caetano FA, Angers S, Ferguson SSG. Ca2+/Calmodulin-dependent protein Kinase II interacts with group I Metabotropic Glutamate and facilitates Receptor Endocytosis and ERK1/2 signaling: role of β-Amyloid. Molecular brain. 2015;8:21. doi: 10.1186/s13041-015-0111-4. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

