Transitioning to active-controlled trials to evaluate cardiovascular safety and efficacy of medications for type 2 diabetes
- PMID: 36002856
- PMCID: PMC9400320
- DOI: 10.1186/s12933-022-01601-w
Transitioning to active-controlled trials to evaluate cardiovascular safety and efficacy of medications for type 2 diabetes
Abstract
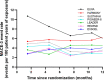
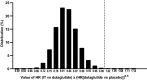
Cardiovascular (CV) outcome trials (CVOTs) of type 2 diabetes mellitus (T2DM) therapies have mostly used randomized comparison with placebo to demonstrate non-inferiority to establish that the investigational drug does not increase CV risk. Recently, several glucagon-like peptide 1 receptor agonists (GLP-1 RA) and sodium glucose cotransporter 2 inhibitors (SGLT-2i) demonstrated reduced CV risk. Consequently, future T2DM therapy trials could face new ethical and clinical challenges if CVOTs continue with the traditional, placebo-controlled design. To address this challenge, here we review the methodologic considerations in transitioning to active-controlled CVOTs and describe the statistical design of a CVOT to assess non-inferiority versus an active comparator and if non-inferiority is proven, using novel methods to assess for superiority versus an imputed placebo. Specifically, as an example of such methodology, we introduce the statistical considerations used for the design of the "Effect of Tirzepatide versus Dulaglutide on Major Adverse Cardiovascular Events (MACE) in Patients with Type 2 Diabetes" trial (SURPASS CVOT). It is the first active-controlled CVOT assessing antihyperglycemic therapy in patients with T2DM designed to demonstrate CV efficacy of the investigational drug, tirzepatide, a dual glucose-dependent insulinotropic polypeptide and GLP-1 RA, by establishing non-inferiority to an active comparator with proven CV efficacy, dulaglutide. To determine the efficacy margin for the hazard ratio, tirzepatide versus dulaglutide, for the composite CV outcome of death, myocardial infarction, or stroke (MACE-3), which is required to claim superiority versus an imputed placebo, the lower bound of efficacy of dulaglutide compared with placebo was estimated using a hierarchical Bayesian meta-analysis of placebo-controlled CVOTs of GLP-1 RAs. SURPASS CVOT was designed so that when the observed upper bound of the 95% confidence interval of the hazard ratio is less than the lower bound of efficacy of dulaglutide, it demonstrates non-inferiority to dulaglutide by preserving at least 50% of the CV benefit of dulaglutide as well as statistical superiority of tirzepatide to a theoretical placebo (imputed placebo analysis). The presented methods adding imputed placebo comparison for efficacy assessment may serve as a model for the statistical design of future active-controlled CVOTs.
Keywords: Antihyperglycemic; Cardiovascular; Imputed placebo; Non-inferiority trial; Type 2 diabetes mellitus.
© 2022. The Author(s).
Conflict of interest statement
DKM reports honoraria, advisory board, and clinical trial executive committee fees from Eli Lilly; fees for consultancy and/or clinical trial executive committee from Boehringer Ingelheim, Sanofi, Novo Nordisk, AstraZeneca, Lexicon, Eisai Inc., Esperion, Metavant, Applied Therapeutics, and Afimmune; clinical trial data monitoring committee fees from Janssen Research and Development LLC, CSL Behring, and GlaxoSmithKline; advisory committee and global advisory board fees from Novo Nordisk and Merck Sharpe and Dohme; and clinical trial scientific advisory committee fees from Pfizer. DD reports research grants and advisory fees from Eli Lilly; research grants from Merck; consulting fees from Intarcia and Sun Pharmaceuticals; and editorial fees from American Diabetes Association journals. SJN reports research support, consulting and personal fees from Eli Lilly; grants and personal fees from AstraZeneca, Amgen, CSL Behring, Esperion, and Sanofi-Regeneron; grants from Anthera, Cerenis, Resverlogix, Novartis, and InfraReDx; and personal fees from Akcea, Boehringer Ingelheim, Kowa, Merck, Takeda, Pfizer, and Novo Nordisk. SEN reports grants from Eli Lilly, AstraZeneca, Esperion Therapeutics, Amgen, and Novo Nordisk. Although SEN works closely with pharmaceutical companies on the development of new therapies for cardiovascular disease, he maintains a longstanding policy of requiring companies to donate all related honoraria directly to charity so that he receives neither income, nor a tax deduction. IP, SS, CRH, JJK and GJW are employees and stockholders of Eli Lilly and Company. JSR was an employee of Eli Lilly and Company.
Figures
References
-
- U.S. Food and Drug Administration. Guidance for industry. Diabetes Mellitus - Evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes. 2008. https://www.federalregister.gov/documents/2008/12/19/E8-30086/guidance-f.... Accessed 12 Feb 2022.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

