Contemporary exploitation of natural products for arthropod-borne pathogen transmission-blocking interventions
- PMID: 36002857
- PMCID: PMC9404607
- DOI: 10.1186/s13071-022-05367-8
Contemporary exploitation of natural products for arthropod-borne pathogen transmission-blocking interventions
Abstract
An integrated approach to innovatively counter the transmission of various arthropod-borne diseases to humans would benefit from strategies that sustainably limit onward passage of infective life cycle stages of pathogens and parasites to the insect vectors and vice versa. Aiming to accelerate the impetus towards a disease-free world amid the challenges posed by climate change, discovery, mindful exploitation and integration of active natural products in design of pathogen transmission-blocking interventions is of high priority. Herein, we provide a review of natural compounds endowed with blockade potential against transmissible forms of human pathogens reported in the last 2 decades from 2000 to 2021. Finally, we propose various translational strategies that can exploit these pathogen transmission-blocking natural products into design of novel and sustainable disease control interventions. In summary, tapping these compounds will potentially aid in integrated combat mission to reduce disease transmission trends.
Keywords: Anti-infectives; Arthropod disease vectors; Disease control; Human pathogen transmission-blocking; Natural products.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

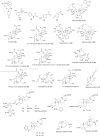
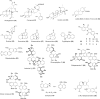
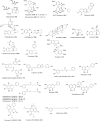

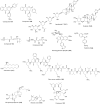
Similar articles
-
Arthropod-borne pathogens of dogs and cats: From pathways and times of transmission to disease control.Vet Parasitol. 2018 Feb 15;251:68-77. doi: 10.1016/j.vetpar.2017.12.021. Epub 2017 Dec 27. Vet Parasitol. 2018. PMID: 29426479 Review.
-
The immunomodulatory factors of bloodfeeding arthropod saliva.Parasite Immunol. 2000 Jul;22(7):319-31. doi: 10.1046/j.1365-3024.2000.00309.x. Parasite Immunol. 2000. PMID: 10886716 Review.
-
Challenges in predicting climate and environmental effects on vector-borne disease episystems in a changing world.J Exp Biol. 2010 Mar 15;213(6):946-54. doi: 10.1242/jeb.037564. J Exp Biol. 2010. PMID: 20190119 Review.
-
Effects of Climate and Climate Change on Vectors and Vector-Borne Diseases: Ticks Are Different.Trends Parasitol. 2016 Aug;32(8):646-656. doi: 10.1016/j.pt.2016.04.015. Epub 2016 May 31. Trends Parasitol. 2016. PMID: 27260548 Review.
-
Modulation of host immunity by haematophagous arthropods.Ann Trop Med Parasitol. 2001 Dec;95(8):755-71. doi: 10.1080/0003498012011118. Ann Trop Med Parasitol. 2001. PMID: 11784430 Review.
References
-
- da Tavares DS, Salgado VR, Miranda JC, Mesquita PRR, de Rodrigues FM, Barral-Netto M, et al. Attraction of phlebotomine sandflies to volatiles from skin odors of individuals residing in an endemic area of tegumentary leishmaniasis. PLoS ONE. 2018;13:e0203989. doi: 10.1371/journal.pone.0203989. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

