Orally administered Odoribacter laneus improves glucose control and inflammatory profile in obese mice by depleting circulating succinate
- PMID: 36002880
- PMCID: PMC9404562
- DOI: 10.1186/s40168-022-01306-y
Orally administered Odoribacter laneus improves glucose control and inflammatory profile in obese mice by depleting circulating succinate
Abstract
Background: Succinate is produced by both human cells and by gut bacteria and couples metabolism to inflammation as an extracellular signaling transducer. Circulating succinate is elevated in patients with obesity and type 2 diabetes and is linked to numerous complications, yet no studies have specifically addressed the contribution of gut microbiota to systemic succinate or explored the consequences of reducing intestinal succinate levels in this setting.
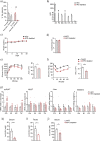
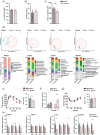
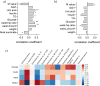
Results: Using germ-free and microbiota-depleted mouse models, we show that the gut microbiota is a significant source of circulating succinate, which is elevated in obesity. We also show in vivo that therapeutic treatments with selected bacteria diminish the levels of circulating succinate in obese mice. Specifically, we demonstrate that Odoribacter laneus is a promising probiotic based on its ability to deplete succinate and improve glucose tolerance and the inflammatory profile in two independent models of obesity (db/db mice and diet-induced obese mice). Mechanistically, this is partly mediated by the succinate receptor 1. Supporting these preclinical findings, we demonstrate an inverse correlation between plasma and fecal levels of succinate in a cohort of patients with severe obesity. We also show that plasma succinate, which is associated with several components of metabolic syndrome including waist circumference, triglycerides, and uric acid, among others, is a primary determinant of insulin sensitivity evaluated by the euglycemic-hyperinsulinemic clamp.
Conclusions: Overall, our work uncovers O. laneus as a promising next-generation probiotic to deplete succinate and improve glucose tolerance and obesity-related inflammation. Video Abstract.
Keywords: Animal models; Glucose tolerance; Inflammation; Obesity; Probiotics; Succinate.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Alegría Ezquerra E, Castellano Vázquez JM, Barrero AA. Obesity, metabolic syndrome and diabetes: cardiovascular implications and therapy. Revista Espanola de Cardiologia. 2008;61(7):752–64. - PubMed
-
- Honda K, Littman DR. The microbiota in adaptive immune homeostasis and disease. Nature. 2016;535(7610):75–84 - PubMed
-
- Spor A, Koren O, Ley R. Unravelling the effects of the environment and host genotype on the gut microbiome. Nat Rev Microbiol. 2011;9(4):279–90 - PubMed
-
- Canfora EE, Meex RCR, Venema K, Blaak EE. Gut microbial metabolites in obesity, NAFLD and T2DM. Nat Rev Endocrinol. 2019;15(5):261–73. - PubMed
-
- Canfora EE, Jocken JW, Blaak EE. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat Rev Endocrinol. 2015;11(10):577–91. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

