Therapeutic effects elicited by the probiotic Lacticaseibacillus rhamnosus GG in children with atopic dermatitis. The results of the ProPAD trial
- PMID: 36003050
- PMCID: PMC9542056
- DOI: 10.1111/pai.13836
Therapeutic effects elicited by the probiotic Lacticaseibacillus rhamnosus GG in children with atopic dermatitis. The results of the ProPAD trial
Abstract
Background: Atopic dermatitis (AD) is a chronic inflammatory skin disease affecting up to 20% of the pediatric population associated with alteration of skin and gut microbiome. Probiotics have been proposed for AD treatment. The ProPAD study aimed to investigate the therapeutic effects of the probiotic Lacticaseibacillus rhamnosus GG (LGG) in children with AD.
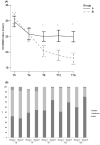
Methods: In total, 100 AD patients aged 6-36 months were enrolled in a randomized, double-blind, controlled trial to receive placebo (Group A) or LGG (1 x 1010 CFU/daily) (Group B) for 12 weeks. The primary outcome was the evaluation of the efficacy of LGG supplementation on AD severity comparing the Scoring Atopic Dermatitis (SCORAD) index at baseline (T0) and at 12-week (T12). A reduction of ≥8.7 points on the SCORAD index was considered as minimum clinically important difference (MCID). The secondary outcomes were the SCORAD index evaluation at 4-week (T16) after the end of LGG treatment, number of days without rescue medications, changes in Infant Dermatitis Quality Of Life questionnaire (IDQOL), gut microbiome structure and function, and skin microbiome structure.
Results: The rate of subjects achieving MCID at T12 and at T16 was higher in Group B (p < .05), and remained higher at T16 (p < .05)The number of days without rescue medications was higher in Group B. IDQOL improved at T12 in the Group B (p < .05). A beneficial modulation of gut and skin microbiome was observed only in Group B patients.
Conclusions: The probiotic LGG could be useful as adjunctive therapy in pediatric AD. The beneficial effects on disease severity and quality of life paralleled with a beneficial modulation of gut and skin microbiome.
Trial registration: ClinicalTrials.gov NCT03863418.
Keywords: butyrate; gut microbiome; infant dermatitis quality of life questionnaire (IDQOL); scoring atopic dermatitis (SCORAD) index; skin microbiome.
© 2022 The Authors. Pediatric Allergy and Immunology published by European Academy of Allergy and Clinical Immunology and John Wiley & Sons Ltd.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures



Similar articles
-
Evaluation of efficacy and safety of Lactobacillus rhamnosus in children aged 4-48 months with atopic dermatitis: An 8-week, double-blind, randomized, placebo-controlled study.J Microbiol Immunol Infect. 2017 Oct;50(5):684-692. doi: 10.1016/j.jmii.2015.10.003. Epub 2015 Nov 27. J Microbiol Immunol Infect. 2017. PMID: 26733351 Clinical Trial.
-
Randomized, placebo-controlled trial of Lactobacillus rhamnosus GG as treatment of atopic dermatitis in infancy.Allergy. 2007 Nov;62(11):1270-6. doi: 10.1111/j.1398-9995.2007.01543.x. Allergy. 2007. PMID: 17919141 Clinical Trial.
-
The Effectiveness of Probiotic Lactobacillus rhamnosus and Lactobacillus casei Strains in Children with Atopic Dermatitis and Cow's Milk Protein Allergy: A Multicenter, Randomized, Double Blind, Placebo Controlled Study.Nutrients. 2021 Apr 1;13(4):1169. doi: 10.3390/nu13041169. Nutrients. 2021. PMID: 33916192 Free PMC article. Clinical Trial.
-
Probiotics for the treatment or prevention of atopic dermatitis: a review of the evidence from randomized controlled trials.Am J Clin Dermatol. 2008;9(2):93-103. doi: 10.2165/00128071-200809020-00002. Am J Clin Dermatol. 2008. PMID: 18284263 Review.
-
Meta-analysis on preventive and therapeutic effects of probiotic supplementation in infant atopic dermatitis.J Dtsch Dermatol Ges. 2023 Aug;21(8):833-843. doi: 10.1111/ddg.15120. Epub 2023 Jun 22. J Dtsch Dermatol Ges. 2023. PMID: 37345893
Cited by
-
Dietary Interventions in Atopic Dermatitis: A Comprehensive Scoping Review and Analysis.Int Arch Allergy Immunol. 2024;185(6):545-589. doi: 10.1159/000535903. Epub 2024 Mar 5. Int Arch Allergy Immunol. 2024. PMID: 38442688 Free PMC article.
-
Recent advances in therapeutic probiotics: insights from human trials.Clin Microbiol Rev. 2025 Jun 12;38(2):e0024024. doi: 10.1128/cmr.00240-24. Epub 2025 Apr 22. Clin Microbiol Rev. 2025. PMID: 40261032 Review.
-
Lactobacillus for the treatment and prevention of atopic dermatitis: Clinical and experimental evidence.Front Cell Infect Microbiol. 2023 Feb 16;13:1137275. doi: 10.3389/fcimb.2023.1137275. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 36875529 Free PMC article. Review.
-
In Vivo Functional Properties of Dairy Bacteria.Microorganisms. 2023 Jul 11;11(7):1787. doi: 10.3390/microorganisms11071787. Microorganisms. 2023. PMID: 37512959 Free PMC article. Review.
-
Probiotics and gut microbiota modulation: implications for skin health and disease management.Arch Microbiol. 2025 Feb 23;207(3):68. doi: 10.1007/s00203-025-04267-6. Arch Microbiol. 2025. PMID: 39988585 Review.
References
-
- Langan SM, Irvine AD, Weidinger S. Atopic dermatitis. Lancet. 2020;396(10247):345‐360. - PubMed
-
- Luger T, Amagai M, Dreno B, et al. Atopic dermatitis: role of the skin barrier, environment, microbiome, and therapeutic agents. J Dermatol Sci. 2021;102(3):142‐157. - PubMed
-
- Park JH, Um JI, Lee BJ, et al. Encapsulated Bifidobacterium bifidum potentiates intestinal IgA production. Cell Immunol. 2002;219(1):22‐27. - PubMed
-
- Krutmann J. Pre‐ and probiotics for human skin. J Dermatol Sci. 2009;54(1):1‐5. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

