Altered high-energy phosphate and membrane metabolism in Pelizaeus-Merzbacher disease using phosphorus magnetic resonance spectroscopy
- PMID: 36003325
- PMCID: PMC9396944
- DOI: 10.1093/braincomms/fcac202
Altered high-energy phosphate and membrane metabolism in Pelizaeus-Merzbacher disease using phosphorus magnetic resonance spectroscopy
Abstract
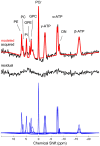
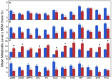
Pelizaeus-Merzbacher disease is an X-linked recessive leucodystrophy of the central nervous system caused by mutations affecting the major myelin protein, proteolipid protein 1. The extent of the altered in vivo neurochemistry of protein, proteolipid protein 1 duplications, the most common form of Pelizaeus-Merzbacher disease, is, however, poorly understood. Phosphorus magnetic resonance spectroscopy is the only in vivo technique that can assess the biochemistry associated with high-energy phosphate and membrane phospholipid metabolism across different cortical, subcortical and white matter areas. In this cross-sectional study, whole-brain, multi-voxel phosphorus magnetic resonance spectroscopy was acquired at 3 T on 14 patients with Pelizaeus-Merzbacher disease with protein, proteolipid protein 1 duplications and 23 healthy controls (all males). Anabolic and catabolic levels of membrane phospholipids (phosphocholine and phosphoethanolamine, and glycerophosphoethanolamine and glycerophosphocholine, respectively), as well as phosphocreatine, inorganic orthophosphate and adenosine triphosphate levels relative to the total phosphorus magnetic resonance spectroscopy signal from 12 different cortical and subcortical areas were compared between the two groups. Independent of brain area, phosphocholine, glycerophosphoethanolamine and inorganic orthophosphate levels were significantly lower (P = 0.0025, P < 0.0001 and P = 0.0002) and phosphocreatine levels were significantly higher (P < 0.0001) in Pelizaeus-Merzbacher disease patients compared with controls. Additionally, there was a significant group-by-brain area interaction for phosphocreatine with post-hoc analyses demonstrating significantly higher phosphocreatine levels in patients with Pelizaeus-Merzbacher disease compared with controls across multiple brain areas (anterior and posterior white matter, superior parietal lobe, posterior cingulate cortex, hippocampus, occipital cortex, striatum and thalamus; all P ≤ 0.0042). Phosphoethanolamine, glycerophosphoethanolamine and adenosine triphosphate levels were not significantly different between groups. For the first-time, widespread alterations in phosphorus magnetic resonance spectroscopy metabolite levels of Pelizaeus-Merzbacher disease patients are being reported. Specifically, increased high-energy phosphate storage levels of phosphocreatine concomitant with decreased inorganic orthophosphate across multiple areas suggest a widespread reduction in the high-energy phosphate utilization in Pelizaeus-Merzbacher disease, and the membrane phospholipid metabolite deficits suggest a widespread degradation in the neuropil content/maintenance of patients with Pelizaeus-Merzbacher disease which includes axons, dendrites and astrocytes within cortex and the myelin microstructure and oligodendrocytes within white matter. These results provide greater insight into the neuropathology of Pelizaeus-Merzbacher disease both in terms of energy expenditure and membrane phospholipid metabolites. Future longitudinal studies are warranted to investigate the utility of phosphorus magnetic resonance spectroscopy as surrogate biomarkers in monitoring treatment intervention for Pelizaeus-Merzbacher disease.
Keywords: Pelizaeus–Merzbacher disease; biochemistry; high-energy phosphate; membrane phospholipids; phosphorus magnetic resonance spectroscopy.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures



Similar articles
-
Altered brain high-energy phosphate metabolism in mild Alzheimer's disease: A 3-dimensional 31P MR spectroscopic imaging study.Neuroimage Clin. 2018 Feb 28;18:254-261. doi: 10.1016/j.nicl.2018.01.031. eCollection 2018. Neuroimage Clin. 2018. PMID: 29876246 Free PMC article.
-
Altered phospholipid metabolism in schizophrenia: a phosphorus 31 nuclear magnetic resonance spectroscopy study.Psychiatry Res. 2013 Dec 30;214(3):365-73. doi: 10.1016/j.pscychresns.2013.06.011. Epub 2013 Sep 14. Psychiatry Res. 2013. PMID: 24045051
-
Quantitative proton MRS of Pelizaeus-Merzbacher disease: evidence of dys- and hypomyelination.Neurology. 2005 Sep 13;65(5):701-6. doi: 10.1212/01.wnl.0000174642.32187.20. Neurology. 2005. PMID: 16157902
-
Pelizaeus-Merzbacher disease and spastic paraplegia type 2: two faces of myelin loss from mutations in the same gene.J Child Neurol. 2003 Sep;18(9):616-24. doi: 10.1177/08830738030180090801. J Child Neurol. 2003. PMID: 14572140 Review.
-
Proton and 31-phosphorus neurospectroscopy in the study of membrane phospholipids and fatty acid intervention in schizophrenia, depression, chronic fatigue syndrome (myalgic encephalomyelitis) and dyslexia.Int Rev Psychiatry. 2006 Apr;18(2):145-7. doi: 10.1080/09540260600581852. Int Rev Psychiatry. 2006. PMID: 16777668 Review.
Cited by
-
Mapping Age Differences in Brain Energy Metabolites and Metabolic Markers of Cellular Membrane Production and Degradation With 31P Magnetic Resonance Spectroscopy.Hum Brain Mapp. 2024 Oct;45(14):e70039. doi: 10.1002/hbm.70039. Hum Brain Mapp. 2024. PMID: 39391993 Free PMC article.
References
-
- Wolf NI, van Spaendonk RML, Hobson GM, Kamholz J. PLP1 disorders. In: Adam MP, Ardinger HH, Pagon RA, eds. GeneReviews University of Washington; 1999.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
