Comprehensive analysis of 7-methylguanosine and immune microenvironment characteristics in clear cell renal cell carcinomas
- PMID: 36003341
- PMCID: PMC9393245
- DOI: 10.3389/fgene.2022.866819
Comprehensive analysis of 7-methylguanosine and immune microenvironment characteristics in clear cell renal cell carcinomas
Abstract
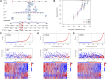
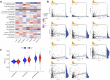
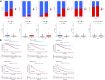
Clear cell renal cell carcinoma (ccRCC) is one of the most common tumors in the urinary system. ccRCC has obvious immunological characteristics, and the infiltration of immune cells is related to the prognosis of ccRCC. The effect of immune checkpoint therapy is related to the dynamic changes of the tumor immune microenvironment (TIM). The 7-methylguanosine (m7G) is an additional mRNA modification ability besides m6A, which is closely related to the TIM and affects the occurrence and development of tumors. At present, the correlations between m7G and the immune microenvironment, treatment, and prognosis of ccRCC are not clear. As far as we know, there was no study on the relationship between m7G and the immune microenvironment and survival of clear cell renal cell carcinomas. A comprehensive analysis of the correlations between them and the construction of a prognosis model are helpful to improve the treatment strategy. Two different molecular subtypes were identified in 539 ccRCC samples by describing the differences of 29 m7G-related genes. It was found that the clinical features, TIM, and prognosis of ccRCC patients were correlated with the m7G-related genes. We found that there were significant differences in the expression of PD-1, CTLA4, and PD-L1 between high- and low-risk groups. To sum up, m7G-related genes play a potential role in the TIM, treatment, and prognosis of ccRCC. Our results provide new findings for ccRCC and help to improve the immunotherapy strategies and prognosis of patients.
Keywords: clear cell renal cell carcinomas; immune checkpoints; immunotherapy; m7G; tumor immune microenvironment.
Copyright © 2022 Xiao, Yang, Yang, Len and Yu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
-
- Albiges L., Tannir N. M., Burotto M., McDermott D., Plimack E. R., Barthelemy P., et al. (2020). Nivolumab plus ipilimumab versus sunitinib for first-line treatment of advanced renal cell carcinoma: extended 4-year follow-up of the phase III CheckMate 214 trial. ESMO Open 5 (6), e001079. 10.1136/esmoopen-2020-001079 - DOI - PMC - PubMed
-
- Antonelli A., Minervini A., Sandri M., Bertini R., Bertolo R., Carini M., et al. (2018). Below safety limits, every unit of glomerular filtration rate counts: assessing the relationship between renal function and cancer-specific mortality in renal cell carcinoma. Eur. Urol. 74 (5), 661–667. 10.1016/j.eururo.2018.07.029 - DOI - PubMed
-
- Bedke J., Albiges L., Capitanio U., Giles R. H., Hora M., Lam T. B., et al. (2021). Updated European association of urology guidelines on renal cell carcinoma: nivolumab plus cabozantinib joins immune checkpoint inhibition combination therapies for treatment-naive metastatic clear-cell renal cell carcinoma. Eur. Urol. 79 (3), 339–342. 10.1016/j.eururo.2020.12.005 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

