Metabolic rewiring directs melanoma immunology
- PMID: 36003368
- PMCID: PMC9393691
- DOI: 10.3389/fimmu.2022.909580
Metabolic rewiring directs melanoma immunology
Abstract
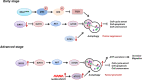
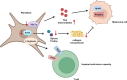
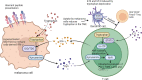
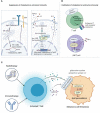
Melanoma results from the malignant transformation of melanocytes and accounts for the most lethal type of skin cancers. In the pathogenesis of melanoma, disordered metabolism is a hallmark characteristic with multiple metabolic paradigms involved in, e.g., glycolysis, lipid metabolism, amino acid metabolism, oxidative phosphorylation, and autophagy. Under the driving forces of oncogenic mutations, melanoma metabolism is rewired to provide not only building bricks for macromolecule synthesis and sufficient energy for rapid proliferation and metastasis but also various metabolic intermediates for signal pathway transduction. Of note, metabolic alterations in tumor orchestrate tumor immunology by affecting the functions of surrounding immune cells, thereby interfering with their antitumor capacity, in addition to the direct influence on tumor cell intrinsic biological activities. In this review, we first introduced the epidemiology, clinical characteristics, and treatment proceedings of melanoma. Then, the components of the tumor microenvironment, especially different populations of immune cells and their roles in antitumor immunity, were reviewed. Sequentially, how metabolic rewiring contributes to tumor cell malignant behaviors in melanoma pathogenesis was discussed. Following this, the proceedings of metabolism- and metabolic intermediate-regulated tumor immunology were comprehensively dissertated. Finally, we summarized currently available drugs that can be employed to target metabolism to intervene tumor immunology and modulate immunotherapy.
Keywords: glycolysis; immunology; immunotherapy; melanoma; metabolism.
Copyright © 2022 Sun, Tian, Chen, Guo and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Melanoma Metabolism: Cell Survival and Resistance to Therapy.Adv Exp Med Biol. 2020;1219:203-223. doi: 10.1007/978-3-030-34025-4_11. Adv Exp Med Biol. 2020. PMID: 32130701 Review.
-
Epigenetics Regulates Antitumor Immunity in Melanoma.Front Immunol. 2022 May 25;13:868786. doi: 10.3389/fimmu.2022.868786. eCollection 2022. Front Immunol. 2022. PMID: 35693795 Free PMC article. Review.
-
Metabolic rewiring in melanoma.Oncogene. 2017 Jan 12;36(2):147-157. doi: 10.1038/onc.2016.198. Epub 2016 Jun 6. Oncogene. 2017. PMID: 27270434 Free PMC article. Review.
-
Signal pathways of melanoma and targeted therapy.Signal Transduct Target Ther. 2021 Dec 20;6(1):424. doi: 10.1038/s41392-021-00827-6. Signal Transduct Target Ther. 2021. PMID: 34924562 Free PMC article. Review.
-
Tumor cell oxidative metabolism as a barrier to PD-1 blockade immunotherapy in melanoma.JCI Insight. 2019 Mar 7;4(5):e124989. doi: 10.1172/jci.insight.124989. eCollection 2019 Mar 7. JCI Insight. 2019. PMID: 30721155 Free PMC article.
Cited by
-
Targeting metabolic reprogramming to overcome immune tolerance in melanoma immunotherapy.Front Immunol. 2025 Jun 5;16:1597770. doi: 10.3389/fimmu.2025.1597770. eCollection 2025. Front Immunol. 2025. PMID: 40539068 Free PMC article. Review.
-
Nutrition and Diet Patterns as Key Modulators of Metabolic Reprogramming in Melanoma Immunotherapy.J Clin Med. 2025 Jun 12;14(12):4193. doi: 10.3390/jcm14124193. J Clin Med. 2025. PMID: 40565936 Free PMC article. Review.
-
Metabolism heterogeneity in melanoma fuels deactivation of immunotherapy: Predict before protect.Front Oncol. 2022 Dec 22;12:1046102. doi: 10.3389/fonc.2022.1046102. eCollection 2022. Front Oncol. 2022. PMID: 36620597 Free PMC article. Review.
-
Metabolic reprogramming in melanoma therapy.Cell Death Discov. 2025 Jul 5;11(1):308. doi: 10.1038/s41420-025-02617-3. Cell Death Discov. 2025. PMID: 40617808 Free PMC article. Review.
-
[Melanoma microenvironment-impact of modern therapies].Dermatologie (Heidelb). 2022 Dec;73(12):907-914. doi: 10.1007/s00105-022-05078-2. Epub 2022 Nov 17. Dermatologie (Heidelb). 2022. PMID: 36394589 Review. German.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

