Electroacupuncture attenuates surgical pain-induced delirium-like behavior in mice via remodeling gut microbiota and dendritic spine
- PMID: 36003380
- PMCID: PMC9393710
- DOI: 10.3389/fimmu.2022.955581
Electroacupuncture attenuates surgical pain-induced delirium-like behavior in mice via remodeling gut microbiota and dendritic spine
Abstract
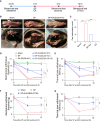
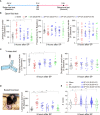
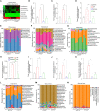
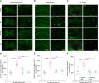
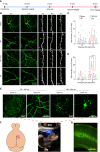
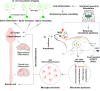
Surgical pain is associated with delirium in patients, and acupuncture can treat pain. However, whether electroacupuncture can attenuate the surgical pain-associated delirium via the gut-brain axis remains unknown. Leveraging a mouse model of foot incision-induced surgical pain and delirium-like behavior, we found that electroacupuncture stimulation at specific acupoints (e.g., DU20+KI1) attenuated both surgical pain and delirium-like behavior in mice. Mechanistically, mice with incision-induced surgical pain and delirium-like behavior showed gut microbiota imbalance, microglia activation in the spinal cord, somatosensory cortex, and hippocampus, as well as an enhanced dendritic spine elimination in cortex revealed by two-photon imaging. The electroacupuncture regimen that alleviated surgical pain and delirium-like behavior in mice also effectively restored the gut microbiota balance, prevented the microglia activation, and reversed the dendritic spine elimination. These data demonstrated a potentially important gut-brain interactive mechanism underlying the surgical pain-induced delirium in mice. Pending further studies, these findings revealed a possible therapeutic approach in preventing and/or treating postoperative delirium by using perioperative electroacupuncture stimulation in patients.
Keywords: delirium; dendritic spine; electroacupuncture; gut microbiota; mice; microglia; surgical pain.
Copyright © 2022 Yang, Ding, Dong, Chen, Zeng, Jiang, Gan, You, Zhao, Zhang, Ren, Wang, Dai, Chen, Zhu, Chen, Shen, Mao and Xie.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The handling editor NT declared a past group authorship with the author ZX.
Figures
Similar articles
-
Electroacupuncture remodels gut microbiota and metabolites in mice with perioperative neurocognitive impairment.Exp Gerontol. 2024 Sep;194:112507. doi: 10.1016/j.exger.2024.112507. Epub 2024 Jul 10. Exp Gerontol. 2024. PMID: 38971546
-
Electroacupuncture inhibits dendritic spine remodeling through the srGAP3-Rac1 signaling pathway in rats with SNL.Biol Res. 2023 May 22;56(1):26. doi: 10.1186/s40659-023-00439-0. Biol Res. 2023. PMID: 37211600 Free PMC article.
-
Electroacupuncture may alleviate neuropathic pain via suppressing P2X7R expression.Mol Pain. 2021 Jan-Dec;17:1744806921997654. doi: 10.1177/1744806921997654. Mol Pain. 2021. PMID: 33626989 Free PMC article.
-
Olfactory Three-Needle Electroacupuncture Improved Synaptic Plasticity and Gut Microbiota of SAMP8 Mice by Stimulating Olfactory Nerve.Chin J Integr Med. 2024 Aug;30(8):729-741. doi: 10.1007/s11655-023-3614-3. Epub 2023 Nov 24. Chin J Integr Med. 2024. PMID: 37999886
-
Electroacupuncture at Tianshu (ST25) and Zusanli (ST36) alleviates stress-induced irritable bowel syndrome in mice by modulating gut microbiota and corticotropin-releasing factor.J Tradit Chin Med. 2022 Oct;42(5):732-740. doi: 10.19852/j.cnki.jtcm.20220719.001. J Tradit Chin Med. 2022. PMID: 36083480 Free PMC article.
Cited by
-
Electroacupuncture alleviates Parkinson's disease by inhibiting the NLRP3 inflammasome pathway.Am J Transl Res. 2025 May 15;17(5):3619-3629. doi: 10.62347/PGKC2376. eCollection 2025. Am J Transl Res. 2025. PMID: 40535642 Free PMC article.
-
Differential effects of electroacupuncture and manual acupuncture on spontaneously hypertensive rats: insights from intestinal microbiota and metabolomics.Front Mol Biosci. 2025 Jul 8;12:1619356. doi: 10.3389/fmolb.2025.1619356. eCollection 2025. Front Mol Biosci. 2025. PMID: 40697389 Free PMC article.
-
P2Y6 promoted pruning of FSTL1 nerves by cutaneous macrophages to reset pain threshold and cardiac function.Purinergic Signal. 2025 Apr 28. doi: 10.1007/s11302-025-10088-5. Online ahead of print. Purinergic Signal. 2025. PMID: 40293604
-
Pathology of pain and its implications for therapeutic interventions.Signal Transduct Target Ther. 2024 Jun 8;9(1):155. doi: 10.1038/s41392-024-01845-w. Signal Transduct Target Ther. 2024. PMID: 38851750 Free PMC article. Review.
-
The possible role of gut microbiota dysbiosis in the pathophysiology of delirium in older persons.Microbiome Res Rep. 2023 May 26;2(3):19. doi: 10.20517/mrr.2023.15. eCollection 2023. Microbiome Res Rep. 2023. PMID: 38046817 Free PMC article. Review.
References
-
- Avidan MS, maybrier HR, Abdallah AB, Jacobsohn E, Vlisides PE, Pryor KO, et al. . Intraoperative ketamine for prevention of postoperative delirium or pain after major surgery in older adults: an international, multicentre, double-blind, randomised clinical trial. Lancet (2017) 390:267–75. doi: 10.1016/S0140-6736(17)31467-8 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

