Pan-Cancer Analysis of the Immunological Role of PDIA5: A Potential Target for Immunotherapy
- PMID: 36003400
- PMCID: PMC9393377
- DOI: 10.3389/fimmu.2022.881722
Pan-Cancer Analysis of the Immunological Role of PDIA5: A Potential Target for Immunotherapy
Abstract
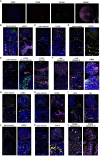
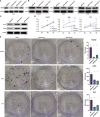
The aberrant protein disulfide isomerase A5 (PDIA5) expression was relevant to the poor prognosis of patients with human cancers. However, its relationship with the epigenetic and genetic alterations and its effect on tumor immunity is still lacking. In the present study, we comprehensively analyzed the immune infiltration role of PDIA5 in human cancers based on large-scale bioinformatics analyses and in vitro experiments. Obvious DNA methylation and moderate alteration frequency of PDIA5 were observed in human cancers. The expression level of PDIA5 was significantly correlated with infiltrated immune cells, immune pathways, and other immune signatures. We found that cancer cells and macrophages exhibited high PDIA5 expression in human cancers using the single-cell RNA sequencing analysis. We also demonstrated the interaction between PDIA5 and immune cells in glioblastoma multiforme (GBM). Multiplex immunofluorescence staining showed the upregulated expression level of PDIA5 and the increased number of M2 macrophage markers-CD163 positive cells in pan-cancer samples. Notably, PDIA5 silencing resulted in upregulated expression of PD-L1 and SPP1 in U251 cells. Silencing of PDIA5 in hepG2 cells, U251 cells, and PC3 cells contributed to a decline in their ability of proliferation, clone formation, and invasion and inhibited the migration of cocultured M2 macrophages. Additionally, PDIA5 also displayed predictive value in the immunotherapy response of both murine and human cancer cohorts. Overall, our findings indicated that PDIA5 might be a potential target for immunotherapies in cancers.
Keywords: bioinformatics; cancer immunotherapy; pan-cancer analysis; protein disulfide isomerase A5; single-cell RNA sequencing.
Copyright © 2022 Chen, He, Chen, Wang, Dai, Liang, Wu, Luo, Zhang, Peng, Zhang, Liu, Zhang, Zhang and Cheng.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

