Evaluation of serological response to anti-SARS-CoV-2 mRNA vaccination in hematological patients
- PMID: 36003404
- PMCID: PMC9393554
- DOI: 10.3389/fimmu.2022.892331
Evaluation of serological response to anti-SARS-CoV-2 mRNA vaccination in hematological patients
Abstract
Introduction: In immunocompromised patients, SARS-CoV-2 mRNA vaccine has been used in Italy from the beginning of the vaccination campaign, but several studies have shown that the serological response of onco-hematological patients was reduced compared to healthy subjects, due to the state of immunosuppression because of both underlying disease and administered therapy.
Methods: We evaluated the association of anti-SARS-CoV-2 spike IgG titers in 215 hematological patients with clinical and demographic variables to verify if it was possible to identify predictive parameters of serological response, as well as using a control group, consisting of healthy health workers of San Carlo Hospital in Potenza. Anti-SARS-CoV2 IgG titers were evaluated after 30-45 days post second dose vaccine using chemiluminescent microparticle immunoassay technology.
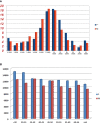
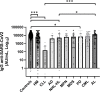
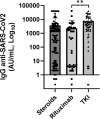
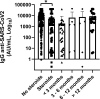
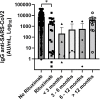
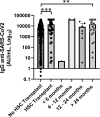
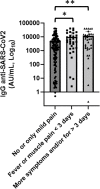
Results: Patients with hematological malignancies, compared with the control arm, had both a mean concentration of anti-SARS-CoV-2 IgG significantly lower and a seroconversion rate numerically lower. All chronic lymphatic leukemia patients showed levels of antibody titer below the mean concentration, also in only clinical surveillance patients. Comparing serological response in hematological malignancies, only acute leukemia patients who were off therapy had the highest seroconversion rate among the patients' cohorts and a mean antibody concentration greater than the control arm. Patients treated with steroids and rituximab showed a lower level of anti-SARS-CoV-2 spike IgG. Differences in anti-spike IgG levels among chronic myeloid leukemia patients stratified according to tyrosine kinase inhibitor therapy and molecular response were observed, and they could have interesting implications on the evaluation of the effects of these drugs on the immune system, but having not reached statistical significance at the moment. The cohort of patients who received a stem cell transplant was very heterogeneous because it included different hematological malignancies and different types of transplant; however, a mean concentration of anti-SARS-CoV2 IgG greater than the control arm was reported. Indeed, among patients who performed a transplant for over 6 months only one had a spike IgG concentration below the cutoff.
Conclusions: Our data confirm reduced serological response in hematological patients after anti-SARS-CoV-2 vaccination. However, we found a great diversity of SARS-CoV-2 antibody response according to types of pathologies and therapies.
Keywords: SARS-COV-2; anti-spike IgG; hematological malignancies; mRNA vaccination; onco-hematology.
Copyright © 2022 Pascale, Nuccorini, Pierri, Di Mare, Fabio, Lerose, Merlino, Schiavo, Amendola, Brucoli, Caputo, Chitarrelli, Cimminiello, Coluzzi, Filardi, Matturro, Vertone, Poggiaspalla, Malaspina, Musuraca, Coralluzzo, Mannarella, Musto, Bellettieri, Martinelli, Cerchione and Pizzuti.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Conti P, Ronconi G, Caraffa A, Gallenga CE, Ross R, Frydas I, et al. . Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J Biol Regul Homeost Agents (2020) 34:327–31. doi: 10.23812/CONTI-E - DOI - PubMed
-
- Spinner CD, Gottlieb RL, Criner GJ, Arribas López JR, Cattelan AM, Soriano Viladomiu A, et al. . Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: A randomized clinical trial. JAMA (2020) 324:1048–57. doi: 10.1001/jama.2020.16349 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

