Allergy, inflammation, hepatopathy and coagulation biomarkers in dogs with suspected anaphylaxis due to insect envenomation
- PMID: 36003410
- PMCID: PMC9393546
- DOI: 10.3389/fvets.2022.875339
Allergy, inflammation, hepatopathy and coagulation biomarkers in dogs with suspected anaphylaxis due to insect envenomation
Abstract
Objectives: To compare concentrations of biomarkers of; allergy [mast cell tryptase (MCT) and histamine], inflammation [interleukin (IL)-6,-10, and-18, CXCL8, CCL2, keratinocyte chemoattractant (KC), C-reactive protein (CRP)], endothelial glycocalyx shedding (hyaluronan), coagulation [prothrombin time, activated partial thromboplastin time, fibrinogen concentration, and von Willebrand Factor antigen, protein C (PC) and antithrombin (AT) activity], and hepatopathy [alanine transaminase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP), and total bilirubin] between dogs with anaphylaxis after suspected insect exposure, dogs with critical illness, and healthy dogs.
Design: This was a single center prospective clinical observational comparative biomarker study that included 25 dogs with anaphylaxis (evidence of insect exposure, acute dermatological signs, and other organ involvement), 30 dogs with other critical illness, and 20 healthy dogs. Differences across groups in biomarker concentrations were tested using one-way ANOVA or Kruskal-Wallis test, with significant P values (<0.05) reported for pairwise differences detected by post-hoc tests. Logistic regression models were used to calculate the area under the receiver operator characteristic curve (AUROC) for discrimination between anaphylaxis and non-anaphylactic illness.
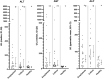
Results: Histamine concentration was significantly higher in the anaphylaxis group than the healthy (P < 0.001) and critically ill groups (P < 0.001), whereas no differences in MCT were detected amongst groups. Biomarker concentrations that were increased relative to healthy dogs in both the anaphylaxis and critically ill groups included IL-10 (P < 0.001 and P = 0.007, respectively), CCL2 (P = 0.007 and P < 0.001, respectively) and AST (both P < 0.001), whereas only the critically ill group had significantly increased CRP (P < 0.001), IL-6 (P < 0.001), KC (P < 0.001), ALP (P < 0.001), and fibrinogen (P = 0.016) concentrations, compared to the healthy group. Only dogs with anaphylaxis had significantly higher hyaluronan (P = 0.021) and ALT (P = 0.021) concentrations, and lower PC (P = 0.030) and AT (P = 0.032) activities, compared to healthy dogs. Both CRP and histamine concentration showed good discrimination between anaphylaxis and other critical illness, with an AUROC of 0.96 (95% CI 0.91-1) and 0.81 (95% CI 0.69-0.93), respectively.
Conclusions: This preliminary study in dogs with anaphylaxis after suspected insect exposure, found evidence of an early innate immune response, glycocalyx shedding and anticoagulant consumption. Both CRP and histamine showed potential clinical utility for differentiation between anaphylaxis and other critical illness.
Keywords: C-reactive protein; antithrombin (AT); canine anaphylaxis; cytokines; histamine; hyaluronan; mast cell tryptase; protein C (PC).
Copyright © 2022 Turner, Boyd, Rossi, Sharp, Claus, Francis and Smart.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
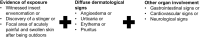



Similar articles
-
Biomarkers of Coagulation and Inflammation in Dogs after Randomized Administration of 6% Hydroxyethyl Starch 130/0.4 or Hartmann's Solution.Animals (Basel). 2022 Oct 6;12(19):2691. doi: 10.3390/ani12192691. Animals (Basel). 2022. PMID: 36230433 Free PMC article.
-
Evaluation of the host cytokine response in dogs with sepsis and noninfectious systemic inflammatory response syndrome.J Vet Emerg Crit Care (San Antonio). 2019 Nov;29(6):593-603. doi: 10.1111/vec.12903. Epub 2019 Oct 21. J Vet Emerg Crit Care (San Antonio). 2019. PMID: 31637812
-
Serum mast cell tryptase measurements: Sensitivity and specificity for a diagnosis of anaphylaxis in emergency department patients with shock or hypoxaemia.Emerg Med Australas. 2018 Jun;30(3):366-374. doi: 10.1111/1742-6723.12875. Epub 2017 Nov 2. Emerg Med Australas. 2018. PMID: 29094472
-
Mechanisms Governing Anaphylaxis: Inflammatory Cells, Mediators, Endothelial Gap Junctions and Beyond.Int J Mol Sci. 2021 Jul 21;22(15):7785. doi: 10.3390/ijms22157785. Int J Mol Sci. 2021. PMID: 34360549 Free PMC article. Review.
-
Biomarkers in Human Anaphylaxis: A Critical Appraisal of Current Evidence and Perspectives.Front Immunol. 2019 Apr 5;10:494. doi: 10.3389/fimmu.2019.00494. eCollection 2019. Front Immunol. 2019. PMID: 31024519 Free PMC article. Review.
Cited by
-
Biomarkers of Coagulation and Inflammation in Dogs after Randomized Administration of 6% Hydroxyethyl Starch 130/0.4 or Hartmann's Solution.Animals (Basel). 2022 Oct 6;12(19):2691. doi: 10.3390/ani12192691. Animals (Basel). 2022. PMID: 36230433 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

