Inflammatory immune response in recipients of transcatheter aortic valves
- PMID: 36003560
- PMCID: PMC9390500
- DOI: 10.1016/j.xjon.2021.02.012
Inflammatory immune response in recipients of transcatheter aortic valves
Abstract
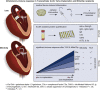
Objective: Transcatheter aortic valve implantation (TAVI) is rapidly replacing cardiac surgery due to its minimal invasiveness and practicality. Midterm immunological studies on the biocompatibility of galactose-alpha-1,3-galactose (α-Gal)-carrying bioprosthetic heart valves for TAVI are not available. In this study we investigated whether bioprosthetic heart valves employed for TAVI augment an α-Gal-specific antibody-dependent and antibody-independent immune response 3 months after TAVI implantation.
Methods: This prospective observational study included 27 patients with severe aortic valve stenosis undergoing TAVI and 10 patients with severe mitral valve regurgitation treated with a transcatheter MitraClip (Abbott Laboratories, Abbott Park, Ill) procedure. Blood samples were drawn before and 90 days after treatment at a routine checkup. Serum samples were analyzed using enzyme-linked immunosorbent assay. Serum concentrations of α-Gal-specific immunoglobulin (Ig) G, IgG subclasses and IgE, complement factor 3a, NETosis-specific citrullinated H3, and the systemic inflammation markers soluble suppression of tumorigenicity and interleukin 33 were evaluated.
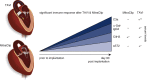
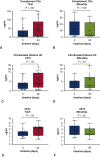
Results: Three months after TAVI, we found significantly increased serum concentrations of α-Gal-specific IgG3, complement factor complement factor 3a, citrullinated H3 levels, and soluble suppression of tumorigenicity (P = .002, P = .001, P = .025, and P = .039, respectively). Sensitization of α-Gal-specific IgE antibodies occurred in 55% of all patients after TAVI.
Conclusions: Our results indicate that TAVI elicits a midterm, specific humoral immune response against α-Gal and causes an unspecific humoral inflammation compared with patients undergoing MitraClip implantation. This observation will lead to a better understanding of postintervention morbidity and the long-term durability of bioprostheses and indicates that caution is appropriate when designing implantation strategies for younger patients.
Keywords: BHV, bioprosthetic heart valve; C3a, complement factor 3a; CitH3, citrullinated H3; DAPI, 4′,6-diamidino-2-phenylindole; ELISA, enzyme-linked immunosorbent assay; IL, interleukin; Ig, immunoglobulin; MitraClip; NET, neutrophil extracellular traps; NETosis; NT-proBNP, N-terminal pro-brain natriuretic peptide; ST2; TAVI; TAVI, transcatheter aortic valve implantation; alpha Gal; bioprosthetic heart valves; complement activation; mAb, monoclonal antibody; sST2, soluble suppression of tumorigenicity-2; α-Gal, galactose-alpha-1,3-galactose.
© 2021 The Author(s).
Figures

References
-
- Baumgartner H., Falk V., Bax J.J., De Bonis M., Hamm C., Holm P., et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2017;38:2739–2791. - PubMed
-
- Siontis G.C.M., Overtchouk P., Cahill T.J., Modine T., Prendergast B., Praz F., et al. Transcatheter aortic valve implantation vs. surgical aortic valve replacement for treatment of symptomatic severe aortic stenosis: an updated meta-analysis. Eur Heart J. 2019;40:3143–3153. - PubMed
-
- Mack M.J., Leon M.B., Thourani V.H., Makkar R., Kodali S.K., Russo M., et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. 2019;380:1695–1705. - PubMed
-
- Konakci K.Z., Bohle B., Blumer R., Hoetzenecker K., Roth G., Moser G., et al. Alpha-Gal on bioprostheses: xenograft immune response in cardiac surgery. Eur J Clin Invest. 2005;35:17–23. - PubMed
-
- Mangold A., Szerafin T., Hoetzenecker K., Hacker S., Lichtenauer M., Niederpoldet T., et al. Alpha-Gal specific IgG immune response after implantation of bioprostheses. Thorac Cardiovasc Surg. 2009;57:191–195. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

