Prediction of neuropeptide precursors and differential expression of adipokinetic hormone/corazonin-related peptide, hugin and corazonin in the brain of malaria vector Nyssorhynchus albimanus during a Plasmodium berghei infection
- PMID: 36003598
- PMCID: PMC9387463
- DOI: 10.1016/j.cris.2021.100014
Prediction of neuropeptide precursors and differential expression of adipokinetic hormone/corazonin-related peptide, hugin and corazonin in the brain of malaria vector Nyssorhynchus albimanus during a Plasmodium berghei infection
Abstract
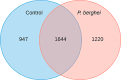
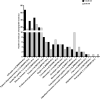
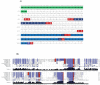
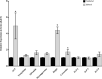
Insect neuropeptides, play a central role in the control of many physiological processes. Based on an analysis of Nyssorhynchus albimanus brain transcriptome a neuropeptide precursor database of the mosquito was described. Also, we observed that adipokinetic hormone/corazonin-related peptide (ACP), hugin and corazonin encoding genes were differentially expressed during Plasmodium infection. Transcriptomic data from Ny. albimanus brain identified 29 pre-propeptides deduced from the sequences that allowed the prediction of at least 60 neuropeptides. The predicted peptides include isoforms of allatostatin C, orcokinin, corazonin, adipokinetic hormone (AKH), SIFamide, capa, hugin, pigment-dispersing factor, adipokinetic hormone/corazonin-related peptide (ACP), tachykinin-related peptide, trissin, neuropeptide F, diuretic hormone 31, bursicon, crustacean cardioactive peptide (CCAP), allatotropin, allatostatin A, ecdysis triggering hormone (ETH), diuretic hormone 44 (Dh44), insulin-like peptides (ILPs) and eclosion hormone (EH). The analysis of the genome of An. albimanus and the generated transcriptome, provided evidence for the identification of myosuppressin neuropeptide precursor. A quantitative analysis documented increased expression of precursors encoding ACP peptide, hugin and corazonin in the mosquito brain after Plasmodium berghei infection. This work represents an initial effort to characterize the neuropeptide precursors repertoire of Ny. albimanus and provides information for understanding neuroregulation of the mosquito response during Plasmodium infection.
Keywords: AKH, adipokinetic hormone; Adipokinetic hormone/corazonin-related peptide; BLAST, Basic Local Alignment Search Tool; CCAP, crustacean cardioactive peptide; CDS, Coding sequence; CNS, central nervous system; EH, eclosion hormone; ETH, ecdysis triggering hormone, Dh44, diuretic hormone 44, ILP, insulin-like peptide; GFP, green fluorescent protein; GO, Gene ontology; GPA2, glycoprotein 2; Hugin; K, lysine; Myosuppressin; NPLP1, neuropeptide-like precursor 1; Neuropeptide precursors; Nyssorhynchus albimanus; PBURS, partner of bursicon; PDH, pigment dispersing hormone; R, arginine; qPCR, quantitative polymerase chain reaction; sNPF, short neuropeptide F.
© 2021 The Authors.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
-
- 201.131.57.23:8080 /Ny. albimanus Brain Study [WWW Document], 2018. URL http://201.131.57.23:8080/nyssorhynchus_albimanus/ (accessed 9.17.18).
-
- Alexa, A., Rahnenfuhrer, J., 2016. topGO: enrichment analysis for Gene Ontology. R Packag. version 2.26.0. R Packag. version 2.26.0. 10.1038/ncomms8832
LinkOut - more resources
Full Text Sources
Research Materials

