Efficacy and safety profile of COVID-19 mRNA vaccine in patients with hematological malignancies: Systematic review and meta-analysis
- PMID: 36003763
- PMCID: PMC9393790
- DOI: 10.3389/fonc.2022.951215
Efficacy and safety profile of COVID-19 mRNA vaccine in patients with hematological malignancies: Systematic review and meta-analysis
Abstract
Patient populations, including those with hematological malignancies, have different responses to COVID-19 vaccines. This study aimed to quantitatively analyze the efficacy and safety of COVID-19 mRNA vaccines in patients with hematological malignancies. Studies reporting on the efficacy and safety of COVID-19 mRNA vaccines in cohorts with hematological malignancies compared to healthy controls were systematically searched in four databases. Meta-analysis and subgroup analyses were performed to generate quantitative synthesis. Fifteen studies with 2,055 cohorts with hematological malignancies and 1,105 healthy subjects as control were included. After two doses of COVID-19 vaccination, only 60% of cohorts with hematological malignancies were seroconverted compared to healthy controls (RR 0.60; 95%CI 0.50-0.71). A single dose of the vaccine resulted in a significantly lower seroconversion rate (RR 0.30; 95%CI 0.16-0.54). Non-Hodgkin lymphoma cohorts had the lowest rate of seroconversion (RR 0.5; 95%CI 0.35-0.71) and those who received active treatments had lower immunological responses (RR 0.59; 95%CI 0.46-0.75). Antibody titers were lower in cohorts with hematological malignancies without any differences in adverse effects in both groups. In conclusion, cohorts with hematological malignancies showed a lower seroconversion rate and antibody titers after receiving COVID-19 mRNA vaccines. The type of malignancy and the status of treatment had a significant impact on the response to vaccination. The vaccines were shown to be safe for both patients with hematological malignancies and healthy controls. Booster doses and stricter health protocols might be beneficial for patient populations.
Keywords: COVID-19; adverse effects; antibody titers; hematologic malignancies; mRNA vaccine; seroconversion rates.
Copyright © 2022 Rinaldi, Pratama, Wiyono, Tandaju, Wardhana and Winston.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
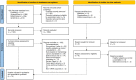
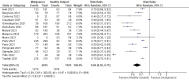
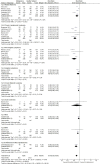
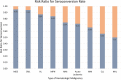


References
-
- World Health Organization . WHO coronavirus (COVID-19) dashboard with vaccination data . Available at: https://covid19.who.int/.
-
- VoPham T, Weaver MD, Hart JE, Ton M, White E, Newcomb PA. Effect of social distancing on COVID-19 incidence and mortality in the US. medRxiv (2020). doi: 10.1101/2020.06.10.20127589 - DOI
-
- Korang SK, von Rohden E, Veroniki AA, Ong G, Ngalamika O, Siddiqui F, et al. Vaccines to prevent COVID-19: A living systematic review with trial sequential analysis and network meta-analysis of randomized clinical trials. PloS One (2022) 17(1):e0260733. doi: 10.1371/journal.pone.0260733 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

