Cuproptosis predicts the risk and clinical outcomes of lung adenocarcinoma
- PMID: 36003780
- PMCID: PMC9393616
- DOI: 10.3389/fonc.2022.922332
Cuproptosis predicts the risk and clinical outcomes of lung adenocarcinoma
Abstract
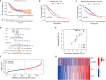
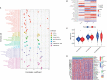
Copper is an essential microelement for the body and a necessary coregulator for enzymatic reactions, yet an unbalanced copper level promotes reactive oxidation and cytotoxicity, which ultimately induces cell death. Several small molecules targeting copper-induced cell death have been investigated, yet few showed promising therapeutic effects in clinical trials. In March 2022, Science first introduced the concept and mechanisms of cuproptosis, suggesting that copper-induced cell death targets the tricarboxylic acid (TCA) cycle via protein lipoylation. Does this novel form of cell death take part in tumorigenesis or tumor progression? Is cuproptosis related to clinical outcomes of diseases? Is there a cuproptosis-related panel for clinical practice in cancer treatment? Herein, based on 942 samples of lung adenocarcinoma (LUAD), we analyzed on gene set level the existence and predictive value of cuproptosis in disease diagnosis and treatment. We screened out and identified the "cupLA" panel which indicates the risk of LUAD occurrence, clinicopathological features of LUAD patients, and could guide clinicians to refine LUAD subtypes and make treatment choices.
Keywords: Copper-induced cell death; chemotherapy sensitivity; clinical outcomes; cuproptosis; immunotherapy sensitivity; lung cancer; tumor microenvironment.
Copyright © 2022 Hu, Wang, Ma, Zhang and Xue.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Cuproptosis-related signature predicts prognosis, immunotherapy efficacy, and chemotherapy sensitivity in lung adenocarcinoma.Front Oncol. 2023 Mar 15;13:1127768. doi: 10.3389/fonc.2023.1127768. eCollection 2023. Front Oncol. 2023. PMID: 37007124 Free PMC article.
-
Molecular subtypes based on cuproptosis-related genes and immune profiles in lung adenocarcinoma.Front Genet. 2022 Oct 12;13:1006938. doi: 10.3389/fgene.2022.1006938. eCollection 2022. Front Genet. 2022. PMID: 36313439 Free PMC article.
-
A novel defined cuproptosis-related gene signature for predicting the prognosis of lung adenocarcinoma.Front Genet. 2022 Aug 15;13:975185. doi: 10.3389/fgene.2022.975185. eCollection 2022. Front Genet. 2022. PMID: 36046242 Free PMC article.
-
The molecular mechanisms of cuproptosis and its relevance to cardiovascular disease.Biomed Pharmacother. 2023 Jul;163:114830. doi: 10.1016/j.biopha.2023.114830. Epub 2023 May 8. Biomed Pharmacother. 2023. PMID: 37150036 Review.
-
Copper homeostasis and cuproptosis in health and disease.Signal Transduct Target Ther. 2022 Nov 23;7(1):378. doi: 10.1038/s41392-022-01229-y. Signal Transduct Target Ther. 2022. PMID: 36414625 Free PMC article. Review.
Cited by
-
Cuproptosis-Related lncRNA Gene Signature Establishes a Prognostic Model of Gastric Adenocarcinoma and Evaluate the Effect of Antineoplastic Drugs.Genes (Basel). 2022 Nov 25;13(12):2214. doi: 10.3390/genes13122214. Genes (Basel). 2022. PMID: 36553481 Free PMC article.
-
A novel Cuprotosis-related signature predicts the prognosis and selects personal treatments for melanoma based on bioinformatics analysis.Front Oncol. 2023 Feb 6;13:1108128. doi: 10.3389/fonc.2023.1108128. eCollection 2023. Front Oncol. 2023. PMID: 36824136 Free PMC article.
-
LncRNA PVT1 promotes cuproptosis through transcriptional activation of FDX1 in colorectal cancer.Redox Biol. 2025 Sep;85:103722. doi: 10.1016/j.redox.2025.103722. Epub 2025 Jun 7. Redox Biol. 2025. PMID: 40505346 Free PMC article.
-
Copper metabolism and cuproptosis in human malignancies: Unraveling the complex interplay for therapeutic insights.Heliyon. 2024 Mar 7;10(5):e27496. doi: 10.1016/j.heliyon.2024.e27496. eCollection 2024 Mar 15. Heliyon. 2024. PMID: 38486750 Free PMC article. Review.
-
Cuproptosis in lung cancer: mechanisms and therapeutic potential.Mol Cell Biochem. 2024 Jun;479(6):1487-1499. doi: 10.1007/s11010-023-04815-y. Epub 2023 Jul 22. Mol Cell Biochem. 2024. PMID: 37480450 Review.
References
LinkOut - more resources
Full Text Sources

